Scientists have revealed how antibiotics can regress certain fast-growing bacteria. The research was published in the journal eLife on June 8th, 2022.
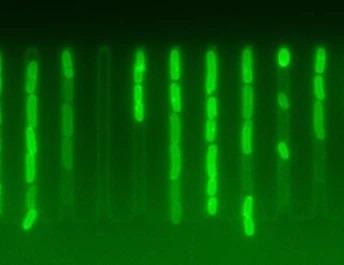 Individual Escherichia coli cells display dramatically different accumulation of antibiotics. Image Credit: Ula Łapińska (CC BY 4.0).
Individual Escherichia coli cells display dramatically different accumulation of antibiotics. Image Credit: Ula Łapińska (CC BY 4.0).
The findings demonstrate that fast-growing individuals in bacterial colonies have much greater levels of active ribosomes, which are protein-synthesizing components within the cell.
This prevents the bacteria from accumulating an essential class of antibiotics known as macrolides and thereby resisting treatment. These results might help scientists build more effective antibiotics that target this coping strategy.
Food poisoning, pneumonia, sepsis, and other deadly disorders can all be caused by bacterial infections. While antibiotics can be used to treat them, their excess consumption in recent years has resulted in bacteria growing increasingly immune to them, posing a serious danger to world health.
Image Credit: fusebulb/Shutterstock.com
To be effective against infection, an antibiotic must reach its cellular target at a concentration high enough to limit bacterial growth.
Antibiotic resistance continues to threaten the viability of current treatments. We need to understand how individual bacteria within a colony can prevent antibiotics from entering their cells, so that we can target this mechanism with new therapies.”
Urszula Łapińska, Postdoctoral Research Assistant, University of Exeter
“Most existing data around drug permeability in bacteria has been attained through measurements that either take an average result from a large population or are derived from a small number of bacteria. This means that little is known about the variability in individual drug accumulation across many single cells in a bacterial colony,” added Łapińska.
To close the gap, Łapińska and her colleagues hypothesized that differences in how bacteria respond to medications could be because of the differences in drug transport rates between individual cells.
To do so, the researchers used a multi-analytical approach that included microfluidics-microscopy, bacteria that pose a health risk such as Escherichia coli, Pseudomonas aeruginosa, Burkholderia cenocepacia, and Staphylococcus aureus, and fluorescent probes derived from antibiotics developed by Dr Mark Blaskovich at the University of Queensland.
This method enabled the researchers to investigate the interactions between conventional antibiotics and a large number of live, individual bacteria in real time, during drug administration.
The scientists gathered data that they could use to swiftly and effectively identify individual bacteria that are antibiotic resistant by combining this strategy with mathematical modeling approaches pioneered by Prof Krasimira Tsaneva-Atanasova from the University of Exeter.
Their findings show that fast-growing individuals in a colony avoid accumulating macrolides within their cells, which contradicts the popular belief that slow cell growth is the most important factor in antibiotic resistance without genetic diversity.
When compared to their slow-growing counterparts, the individuals had a considerably larger number of ribosomes before medication treatment, allowing them to avoid the drug. Ribosomes are required for several physiological functions, including efflux, which is a mechanism that removes hazardous molecules like antimicrobial compounds from the cell.
The researchers then demonstrated that chemically modifying the outer membrane of bacterial cells allows for the eradication of fast-growing variations with minimal macrolide accumulation, thereby assisting in the battle against antibiotic resistance.
This work reveals a hitherto unrecognized survival strategy in some members of bacterial colonies. These insights will directly benefit microbiologists and clinicians who are working on the development of more effective antibiotic therapies.”
Dr Stefano Pagliara, Senior Lecturer, Microfluidics, University of Exeter
“In the longer term, we hope that using our novel approach in clinical settings will help inform the design of improved drugs and help us in the fight against antibiotic resistance,” concluded Dr Pagliara.
Source:
Journal reference:
Łapińska, U., et al (2022) Fast bacterial growth reduces antibiotic accumulation and efficacy eLife. doi:10.7554/eLife.74062