Immunotherapy has become an exciting advancement in medicine, with these therapies aiming to strengthen patients' immune systems through an approach known as the 'fifth pillar' of cancer treatment. These can have the ability to shrink and eliminate advanced tumors and can include immune checkpoint inhibitor drugs and CAR-T cells. The emergence of CAR-T cells has shown the ability to eliminate advanced leukemia and lymphoma and has the potential to be revolutionary for treating infectious diseases.
Challenges For T Cells
Two broad subsets of T cells are involved with actions for specific and long-term immunity, with effector T cells working against pathogens and cancerous cells, while Treg cells protect the body from autoimmune responses.
Although these cells are potent for engaging against foreign bodies, they can sometimes be ineffective for certain infectious diseases and tumors that have escape mechanisms, allowing them to bypass the immune system. This is also similar for autoimmune diseases such as type 1 diabetes, which illustrates the challenge of Treg cells in protecting the host against these immune responses from the self. Additionally, this also occurs in organ transplantation, where these cells are unable to protect tissues against immune rejection against the new organ that is transplanted.
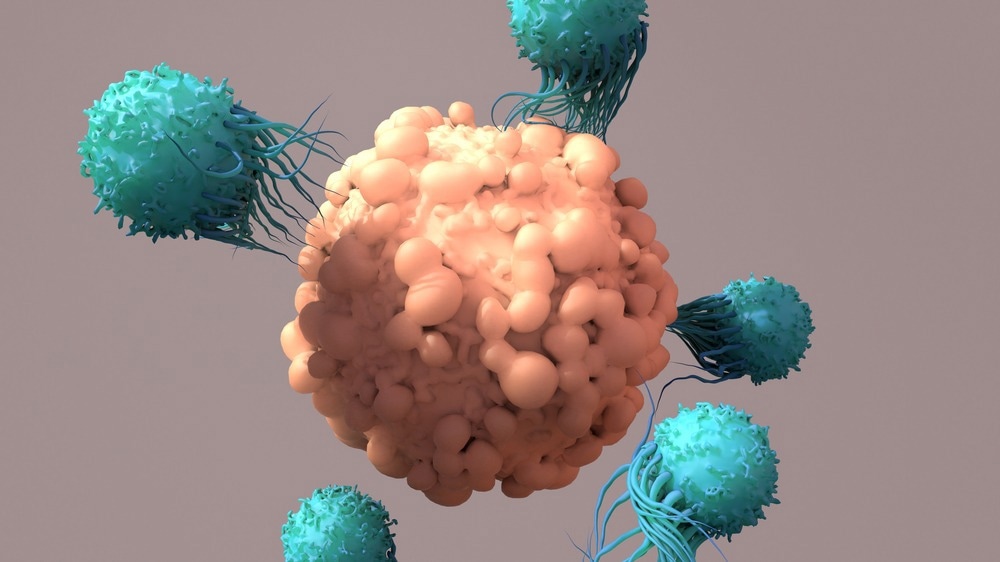
Image Credit: Design_Cells/Shutterstock.com
What Are CAR-T Cells?
Using immunotherapy, such as through CAR-T cells, may be a novel approach to protecting against infectious diseases.
Chimeric antigen receptor (CAR) T cells are genetically engineered lymphocytes producing a chimeric antigen receptor directed towards tumor cell antigens. Their structure consists of an extracellular target antigen-binding domain, a hinge region, a transmembrane domain, and one or more intracellular signaling domains.
CAR-T cells can overcome barriers that are faced by native T cells in the body and work by altering the way T cells recognize antigens with direct binding to cell surface proteins that do not require the usual peptide presentation by MHC molecules; this enables T cell activation and can lead to the elimination of cells.
These engineered lymphocytes have more specificity in targeting, no HLA restriction, and avoiding T cell escape mechanisms used by infectious pathogens and cancer cells that are ineffective to conventional immune responses.
Similar to other immunotherapy approaches, such as drugs, including immune checkpoint inhibitors, CAR-T cell therapy demonstrates their potential to eliminate advanced diseases with the ability to maintain cancer recovery. There have been six CAR-T cell therapies approved by the Food and Drug Administration (FDA) since 2017, and these have been for the application of blood cancers such as lymphomas, certain leukemia disorders, and multiple myeloma.
How CAR-T Cell Therapy Works
CAR-T cell therapy consists of taking a blood sample from patients to remove the T cells, which are then genetically engineered within a laboratory setting; the gene for the CAR is then inserted into T cells, and the CAR protein is expressed on the surface of the cells. This ultimately forms the CAR-T cells, which are reproduced and expanded in the laboratory to create millions and then returned back to the patient through intravenous infusion.
At this point, these cells are equipped to bind to antigens on cancer cells to eradicate them.
While these special cells were initially developed as a novel treatment for the most common cancer found in children, acute lymphoblastic leukemia (ALL), they can also be used for various infectious diseases.
This can include the potential of clearing the human immunodeficiency virus (HIV). This would be significant as a small portion of these cells are able to evade immunosurveillance and remain hidden from the immune system due to a lack of expression of viral antigens. These cells can remain dormant and start to produce the infectious virus spontaneously after several years, requiring infected patients to need life-long antiretroviral therapy.
Using CAR-T cells against this imposing virus may alleviate the obstacles faced by the body's own natural virus-specific cytotoxic T lymphocytes (CTLs). While HIV viral cells are able to use escape mechanisms against CTLs through mutations within CTL-targeted epitopes that make these cells invisible to the immune response, this is less likely to occur with HIV-specific CAR-T cells, and with millions of these cells being produced before insertion into the body, the immune response can be strengthened.
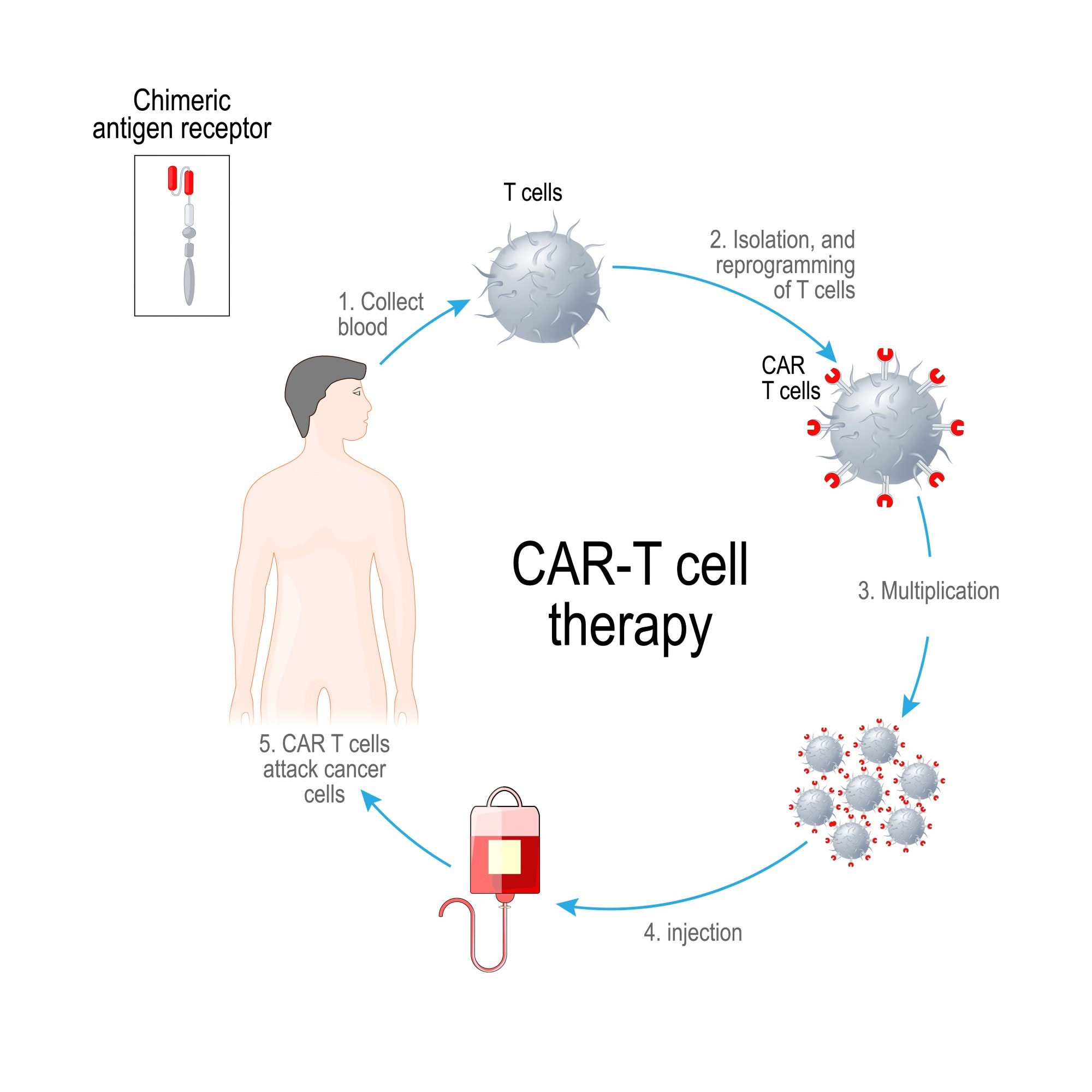
Image Credit: Designua/Shutterstock.com
The Future of Fighting Infectious Diseases
The use of CAR-T cells for infectious diseases, from leukemia to HIV, has illustrated its potential to be used for other diseases that may require a larger immune response from the body against resistant pathogens.
Research into these uses has further proven the versatility of CAR-T cells, which may become a novel approach to advancing precision or personalized medicine. However, there are still challenges to overcome with this newly emerging method, such as targeting multiple antigens or targeting tumor-restricted post-translational modifications, and innovative strategies for these are still underway.
The promising future of research in this area of medicine is bright, with FDA approvals being provided for emerging CAR-T cell therapies. Developing novel treatment approaches using CAR-T cells for more infectious diseases may even further revolutionize this field.
Sources:
- National Cancer Institute. 2022. NCI Dictionary of Cancer Terms. [online] Available at: <https://www.cancer.gov/publications/dictionaries/cancer-terms/def/car-t-cell-therapy> [Accessed 13 March 2022].
- National Cancer Institute. 2022. CAR T Cells: Engineering Immune Cells to Treat Cancer. [online] Available at: <https://www.cancer.gov/about-cancer/treatment/research/car-t-cells> [Accessed 13 March 2022].
- Sterner, R. and Sterner, R., 2021. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer Journal, 11(4). https://doi.org/10.1038/s41408-021-00459-7
- Maldini, C., Ellis, G. and Riley, J., 2018. CAR T cells for infection, autoimmunity and allotransplantation. Nature Reviews Immunology, 18(10), pp.605-616. https://doi.org/10.1038/s41577-018-0042-2
- Stewart, A. and Henden, A., 2021. Infectious complications of CAR T-cell therapy: a clinical update. Therapeutic Advances in Infectious Disease, 8, p.204993612110367. https://doi.org/10.1177/20499361211036773
Further Reading