Popularity of the more traditional cannabis flower has been overshadowed by demand for cannabis concentrate products, for example, extracts, edibles, tinctures, waxes and oils legally manufactured for both recreational and medicinal purposes.
The majority of concentrates are extracted via solvents including supercritical CO2, propane, butane, hydrocarbons, alcohol or water. The solvents’ role is to extract terpenes and cannabinoids from plant material.
In some instances, impurities from the solvent and the solvent itself are left behind in the extracted material - residual solvents that are by-products of the extraction process. These impurities can be toxic under certain circumstances, meaning that residual solvent analysis is an essential component of cannabis testing.
A combination of headspace (HS) gas chromatography (GC) and mass spectrometry (MS) detection remains the most common method for measuring residual solvents. This method ensures that false positives are not reported.
Headspace’s key advantages lie within its potential as a straightforward, accurate and precise technique that allows components of interest (for example, terpenes and residual solvents) to be introduced into the analytical system.
Non-volatile matrix components remain in the sample vial. Because these do not enter the GC, the system is able to offer rapid analysis with minimal maintenance requirements.
The technique is well established, and is already recognized as an appropriate means of quantitation in a number of regulated industries where results are routinely required to stand up to scrutiny in legal proceedings, for example, pharmaceutical forensics, food and environmental applications.1,2,3,4
It should be noted that there are currently no federal regulations in the US - allowable concentration limits for residual solvents are specified by individual states or the country where the cannabis is grown.
Table 1 provides an example of proposed residual solvents and action levels for cannabis products in the State of California.5
The study detailed in this article focuses on the analysis of residual solvents using pressure-balanced headspace (HS) sample introduction coupled with Gas Chromatography/Mass Spectrometry (GC/MS).
It will also explore the importance of maximizing sample throughput while ensuring the unambiguous separation of all compounds.
Table 1. List of proposed residual solvents and action limits in cannabis products for the State of California. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| Compound |
CA Action Levels (ppm) |
| Propane |
1000 |
| Butane |
1000 |
| Methanol |
600 |
| Ethylene Oxide |
1 |
| Pentane |
1000 |
| Ethanol |
1000 |
| Ethyl Ether |
1000 |
| Acetone |
1000 |
| Isopropyl Alcohol |
1000 |
| Acetonitrile |
80 |
| Methylene Chloride |
1 |
| Hexane |
60 |
| Ethyl Acetate |
1000 |
| Chloroform |
1 |
| Benzene |
1 |
| 1,2-Dichloroethane |
1 |
| Heptane |
1000 |
| Trichloroethylene |
1 |
| Toluene |
180 |
| Xylenes total |
430 |
Instrumentation
The analysis was completed using the TurboMatrix™ HS sampler and a Clarus® SQ 8 GC/MS (PerkinElmer Inc., Shelton, CT).
The widely recognized advantages of headspace sampling stem from its role as a separation technique in which volatile material (for example, residual solvents and terpenes) is extracted from a heavier sample matrix before being directly injected into a GC for analysis.6
The key benefit of utilizing MS detection is that several organic compounds associated with residual solvents will elute simultaneously (co-elute). This means that the unique mass spectrum of each compound can be separated and detected without the need to use additional detectors.
Employing this approach to the identification and quantification of residual solvents facilitates more rapid analysis, improved productivity, faster product release and increased return on investment.
Experimental
Sample preparation after extraction
A number of states require analysts to utilize multiple sampling points from non-homogenous samples (for example, edibles and waxes) in order to ensure that analyzed samples are suitably representative.
A total of five sampling points from one sample are recommended in states where this requirement is in place.
For example, if the regulatory amount for testing is 500 mg is then a total of 5 x 100 mg portions should be inserted into a vial before being filled to a final volume of 10 mL with dimethylacetamide (DMA).
A total of 20 µL of diluent should then be placed into the HS vial prior to this being capped and inserted onto the HS autosampler for analysis.
In cases where there is no requirement for average sampling, a 40 mg aliquot of the extract can be directly weighed into the HS vial before being capped and placed onto the autosampler tray. This is the preferred and most straightforward approach.
A Validatable Solution
A rapid, robust and accurate GC/MS-HS solution and associated SOP were developed in order to separate out required analyte compounds in each of the concentrates being tested.
Mass and/or time domains were used to identify the target compound before specific compounds were quantified using commercially available standards from Emerald Scientific (San Luis Obispo, CA):
- California Residual Solvent Mix #1 (Inhalation) reference number STRS01102
- California Residual Solvent Mix #2 (Inhalation) reference number STRS01103
Chromatographic separation of residual solvents is demonstrated in Figure 1.
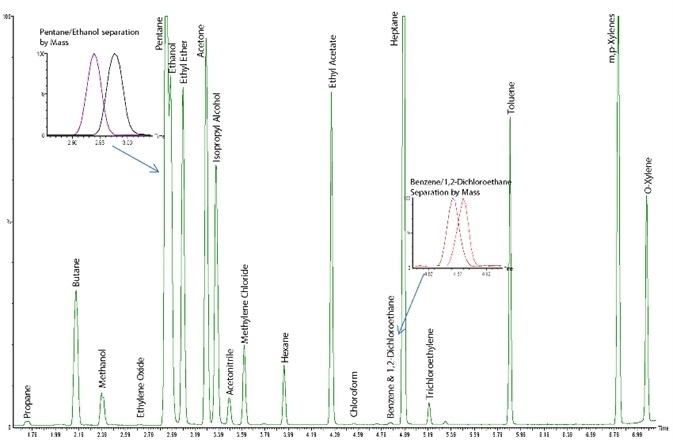
Figure 1. Chromatographic separation of all the compounds listed in Table 1. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Chromatographic peaks of two pairs of co-eluting compounds (pentane/ethanol at around 3.0 minutes and benzene/1,2-dichloroethane at around 4.8 minutes) are highlighted to demonstrate the benefits of detection via mass spectrometry.
This figure explicitly shows how these compounds have been further separated by mass. It took around 7.5 minutes to elute and identify all compounds.
In order to represent the necessary quantitation ranges and meet the required action levels, a multi-level concentration suite of standards was prepared.
Repeatability studies were performed by preparing eight vials with 20 µL of the same concentration standard. Table 2 displays data that validates the precision and linearity attained via this method. It also includes California action levels and method reporting limits.
Table 2. Linearity and precision achieved using this method, together with the reporting and California action limits for cannabis products. Note: Reporting limits are based on a 1 to 20 sample dilution; therefore if no dilution is carried out, reporting limits are 20x lower. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| Compound |
Corelation
Coefficient |
Precision
(n=8) |
CA Action
Levels (ppm) |
PerkinElmer Reporting
Levels (ppm)* |
| Propane |
0.9996 |
2.30 |
1000 |
3.20 |
| Butane |
0.9991 |
1.08 |
1000 |
57.60 |
| Methanol |
0.9996 |
1.29 |
600 |
19.20 |
| Ethylene Oxide |
0.9994 |
2.29 |
1 |
1.00 |
| Pentane |
0.9997 |
1.95 |
1000 |
14.40 |
| Ethanol |
0.9997 |
1.41 |
1000 |
19.20 |
| Ethyl Ether |
0.9998 |
0.59 |
1000 |
9.60 |
| Acetone |
1.0000 |
0.94 |
1000 |
14.40 |
| Isopropyl Alcohol |
0.9996 |
1.33 |
1000 |
9.60 |
| Acetonitrile |
0.9998 |
0.45 |
80 |
1.16 |
| Methylene Chloride |
0.9999 |
1.08 |
1 |
1.00 |
| Hexane |
0.9996 |
1.08 |
60 |
0.48 |
| Ethyl Acetate |
0.9999 |
1.02 |
1000 |
6.80 |
| Chloroform |
0.9996 |
1.68 |
1 |
0.60 |
| Benzene |
1.0000 |
1.02 |
1 |
0.96 |
| 1,2-Dichloroethane |
0.9993 |
2.15 |
1 |
0.96 |
| Heptane |
0.9997 |
1.08 |
1000 |
9.60 |
| Trichloroethylene |
0.9998 |
2.12 |
1 |
0.48 |
| Toluene |
0.9998 |
1.46 |
180 |
2.88 |
| Xylenes total |
0.9999 |
0.86 |
430 |
2.88 |
Discussion of Results
The chromatographic peaks exhibit a good degree of separation with a runtime of around 7.5 minutes and a sample-to-sample cycle time of less than 11 minutes (Figure 1).
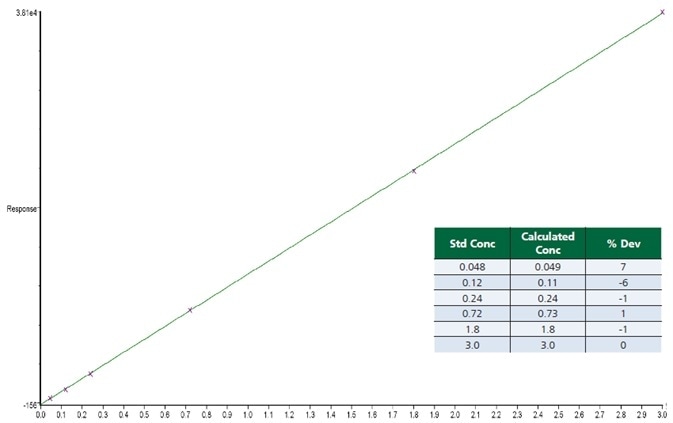
Figure 2. Calibration curve for benzene, showing the % deviation for each standard. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Mass spectrometry enables the rapid identification of components without the risk of false positives.
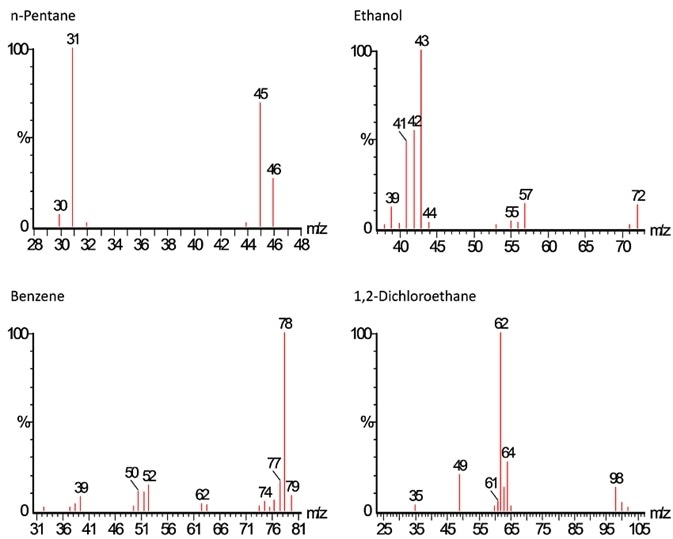
Figure 3. The mass chromatogram of two pairs of compounds that co-elute in time, ethanol/pentane and benzene/1,2- dichloroethane, show they have very unique spectra and quantitation ions. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
The two pairs that co-elute in time (ethanol/pentane and benzene/1,2- dichloroethane) exhibit distinct spectra and quantitation ions (Figure 3). Interference-free integration and quantitation is possible because quantitation is completed on the mass chromatogram (or quantitation ion) of the unique mass.
This would not be viable if the analysis was performed using flame ionization detection.
Figure 2 features an example calibration curve for the target compound benzene. The table in this figure highlights the quantitation of each point using this curve. The % deviation calculated for each point exhibits a very strong correlation with the calibration standards.
Table 2 demonstrates excellent linearity across the compound range achievable via a multi-level calibration. All targets are confirmed to possess a correlation coefficient value better than 0.9993. This turnkey solution is also highly precise, featuring a relative standard deviation of less than 2.3 % for every individual compound.
It should be noted that reporting limits are based upon a 1:20 sample dilution factor. This means that the reporting limit may be further improved by using smaller – or even no - dilutions.
Conclusions
The examples presented here demonstrate the potential of headspace technology coupled with GC/MS for the identification and measurement of residual solvents in a diverse array of products employing cannabis concentrates.
Headspace sample introduction offers a range of advantages over other approaches: it requires minimal sample preparation and very little operator interaction.
The combination of headspace sampling and GC/MS enables rapid, interference-free, unambiguous integrations. There is also minimal risk of false positives.
MS also affords analysts the ability to identify unknown components that may be present in the sample, even if these are not target compounds.
This is a considerable advantage over a single detector such as flame ionization detection (FID) whereby the simultaneous elution of a non-targeted compound and a targeted compound would return a result over the action limit, a failed batch and a cannabis product that is unable to be sent to the market.
It should be highlighted that a GC/MS-HS method is rapid and able to quantify residual solvents in all concentrate samples and required matrices. It also offers essentially maintenance-free operation, while enhancing overall productivity and bolstering a laboratory’s wider business operations.
References
- Residual Solvents in Pharmaceuticals by USP Chapter <467> Methodology, David Scott, PerkinElmer Inc. Application Note, https://www.perkinelmer.com/lab-solutions/resources/docs/ APP_Residual_Solvents_In_Pharmaceuticals_by_ USP_467_013617_01.pdf.
- Increasing Accuracy of Blood-Alcohol Analysis Using Automated Headspace-Gas Chromatography, John Musselman, Anil Solanky, William Arnold, PerkinElmer Inc.
- Measuring Environmental Volatile Organic Compounds Using US EPA Method 8260B with headspace trap GC/MS, Heidi Griffith, PerkinElmer Inc. Application Note, http://www.perkinelmer. com/lab-solutions/resources/docs/APP_ GasChromaUSEPA8260B.pdf.
- Monitoring Volatile Organic Compounds in Beer Production Using the Clarus SQ 8 GC/MS and TurboMatrix Headspace Trap Systems, Lee Marotta, Andrew Tippler, PerkinElmer Application Note, http://www.perkinelmer.com/PDFs/ Downloads/App_FoodBeerVolatileCompounds.pdf.
- California Bureau of Cannabis Control – Proposed Text of Regulations, July, 2018; https://cannabis.ca.gov/.
- An Introduction to Headspace Sampling Gas Chromatography Fundamentals and Theory: Andrew Tippler, PerkinElmer Inc. Application Note, https://www.perkinelmer.com/.
Acknowledgments
Produced from materials originally authored by Lee Marotta, Tom Kwoka, David Scott, Miles Snow and Toby Astill from PerkinElmer.
About PerkinElmer Cannabis & Hemp Testing Solutions
 With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
They help drive analytical best practices and operating procedures and commit to ensuring your laboratory has maximum uptime. Learn about their various instruments, testing methods, and applications for cannabis analyses. Let them work with you to build an efficient workflow, so you can focus on growing your business.
Sponsored Content Policy: AZO Life Science publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZO Life Science, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.