The therapeutic benefits of medical cannabis for ailments such as multiple sclerosis, cancer and ALS has led to its legalization in over 50% of the US.1,2,3
Much like established agricultural crops, cannabis cultivation will occasionally involve the use of pesticides to improve growth yield and protect plants from pests. Residue from prohibited pesticides such imidacloprid, myclobutanil, etoxazole, abamectin and spiromesifen have been detected on cannabis flowers and concentrated further in edibles and extracts. Prolonged exposure to these and other pesticides can cause serious health risks in humans, thus the analysis of pesticides in cannabis is an important element of consumer safety.
A high proportion of cannabis products have recently been found to be affected by elevated levels of pesticide residue, leading to public safety alerts and product recalls. For example, in October 2015, a case in Colorado lead to the recalling pf 20,000 packages of cannabis flowers due to pesticide contamination. In November 2016, another case caused Oregon officials to issue a health alert for certain batches of cannabis.
A large number of today’s cannabis products are inhaled after combusting them. This has led to growing concern among regulators and consumers about the unknown effects of pesticide compounds inhaled as part of the cannabis consumption process.4,5
Pesticides are not the only concern: cultivation conditions for cannabis are also favorable to mold and fungus growth, which can lead to the presence of carcinogenic mycotoxins, including aflatoxins and ochratoxin A.
Testing of mycotoxins and pesticide levels in cannabis is essential in ensuring quality control and consumer safety.
The use of high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) has become the preferred procedure for mycotoxin and pesticide analysis for a number of reasons, including:
- Its superior selectivity
- Its ruggedness
- Its high levels of sensitivity
- Its minimal sample preparation requirements
While methods for gas chromatography-mass spectrometry (GC-MS/MS) have been developed for the analysis of pesticides in cannabis samples, these methods can only be utilized in a smaller subset number of analytes.
For example, compounds like abamectin, which has a high molecular weight, or daminozide, which is highly polar, are not suitable for analysis by GC-MS/MS.
This is due to them being heat labile and at risk of degradation in either the GC injection port or the column at elevated temperatures.
Procedures utilizing GC-MS/MS are not as robust as LC-MS/MS methods for pesticide analysis in complex matrices. This is due to the extensive sample preparation requirements necessary to avoid GC injection port contamination from the complex matrices.6,7
In the US, there are no federal guidelines for analyzing pesticides in cannabis samples. As a result, US states have developed their own testing guidelines.
For example, Oregon was the first state in the US to create thorough guidelines for analyzing pesticide residues in cannabis. It also set regulatory limits for 59 pesticides in cannabis.8
In contrast, the state of California issued more strict action limits for 66 pesticides – a list that includes all but one of those found in Oregon’s list and another eight - plus five mycotoxin residues found in cannabis flowers and edibles.9
Many studies on the analysis of pesticides in cannabis have been published, but these contain certain deficiencies.10,11,12 For example, many of these studies do not reach the detection limits required to meet the state of California’s action limits.
They also utilize time-consuming sample preparation methods - such as QuEChERS with dSPE - with poor recoveries for some of the pesticides.
This means both LC-MS/MS and GC-MS/MS based instruments need to be utilized for the analysis of all the pesticides, causing substantial increases in the analysis’ complexity, cost and turnaround time.
The application development team at PerkinElmer analyzed all 66 pesticides - including very chlorinated and hydrophobic pesticides normally analyzed by GC-MS/MS – as well as five mycotoxins spiked in cannabis flower extracts well below the action limits stipulated by California’s guidelines.
An LC-MS/MS instrument was utilized with APCI and ESI sources. A simple solvent extraction method with excellent recoveries for all analytes in an acceptable range of 70-120% was also utilized in the analysis.
Experimental
Hardware and Software
Chromatographic separation was conducted on a PerkinElmer LC-MS/MS QSight® LX50 UHPLC system.
Detection was attained utilizing a PerkinElmer QSight 220 MS/MS detector with a dual ionization APCI and ESI source, which operate independently with two individual inlets.
The instrument control, data acquisition and data processing procedures were carried out using the Simplicity™ 3Q software platform.
Sample Preparation Method
The sample preparation procedure (with 10-fold dilution) was carried out as follows:
- Approximately 5 g of cannabis flower was taken as a representative of each sample batch, then ground finely.
- A total of 1 g of the sample was measured and placed into 50 ml centrifuge tube.
- A total of 10 µl measure of the internal standard solution was spiked.
- Three steel balls with a diameter of 10 mm were placed in the tube to allow for efficient extraction during vortex mixing.
- A 5 ml measure of LC/MS grade acetonitrile was placed in the tube, which was then capped.
- The tube was placed on a multi-tube vortex mixer and allowed to vortex for 10 minutes. The extract was centrifuged in the tube for 10 minutes at 3000 rpm.
- The solvent was filtered into a 5 ml glass amber vial using 0.22 μm nylon syringe-filter and cap the vial.
- The vial was marked the relevant sample ID.
- A total of 0.5 ml of extracted sample was moved into a 2 mL HPLC vial.
- This was diluted with 0.5 ml of LC/MS grade acetonitrile and the solution was mixed.
- Lastly, 3 µl of the sample was injected for LC-MS/MS analysis, utilizing pesticide methods.
LC Method and MS Source Conditions
The parameters for LC method and MS source are displayed in Table 1.
Table 1. LC Method and MS Source Conditions. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| LC Conditions |
| LC Column |
PerkinElmer Quasar Pesticide Column (4.6 × 100 mm, 2.7 μm) Part Number: N9306880 |
Mobile Phase A
(ESI method) |
2 mM ammonium formate + 0.1% formic acid (in water) |
Mobile Phase B
(ESI method) |
2 mM ammonium formate + 0.1% formic acid (in methanol) |
Mobile Phase
Gradient |
A 18.5 min. (this time includes both analysis time and column equilibration time) LC-MS/MS method with optimized gradient using ESI source was used for separation and analysis of 63 out of 66 pesticides and five mycotoxins residues at low levels in cannabis matrix with minimal matrix interference. A fast 6 min. LC-MS/MS method with short gradient, optimum mobile phase composition and APCI source was used for measurement of remaining three pesticides. |
Column Oven
Temperature |
30 ºC |
Auto sampler
Temperature |
10 ºC |
| Injection Volume |
3.0 μL for LC-MS/MS method with ESI source.
10 μL for LC-MS/MS method with APCI source. |
| MS Source Conditions for ESI Source and APCI Source |
ESI Voltage
(Positive) |
+5500 V |
ESI Voltage
(Negative) |
-4200 V |
APCI Corona
Discharge |
-5 μA |
| Drying Gas |
120 arbitrary units |
| Nebulizer Gas |
350 arbitrary units |
| Source Temperature |
315 ºC |
| HSID Temperature |
200 ºC |
| Detection mode |
Time-managed MRM™ |
Results and Discussion
The range of pesticides analyzed in this study included polar and non-polar compounds. As a result, 100% acetonitrile was utilized to obtain all the analytes from the samples.
The cannabis matrix is hydrophobic, so further dilution of the extract was carried out with the aqueous mobile phase to ensure it was compatible with the reverse phase column. Precipitation meant that this protocol resulted in lower recoveries of some pesticides.
To obtain a higher performing method, the cannabis extracts were diluted with acetonitrile by a factor of 10 to achieve higher pesticides recovery and reduce matrix effects.
The reverse phase LC method utilizes an aqueous mobile phase at the start of the LC run to allow for better retention of the polar compounds on the column.
The injection of an organic solvent, for example, an acetonitrile sample, on the LC results in poor chromatographic peaks for early eluting polar compounds. A sample injection volume of 2 ml was used in this study to overcome this issue.
The analysis of pesticides in cannabis can be taxing as its matrix composition is complex. It also comprises compounds from a range of classes such as cannabinoids, sugars, flavonoids, fatty acids, terpenes, hydrocarbons and others.
The key concern in utilizing LC-MS/MS is the sample matrix effect, as it results in variable signal ion suppression and matrix interference.
Quantification of pesticide residues in cannabis is complicated due to the disparity in high concentration levels of naturally occurring cannabinoids as well as high terpene content.
In this study, a generic extraction method with dilution was utilized, the best MRM transitions were selected, and the LC gradient was optimized to facilitate low level pesticide analysis with good recovery in a complex cannabis matrix.
The analysis of pesticides in cannabis samples and other food matrices is usually carried out utilizing both the GC-MS/MS and LC-MS/MS method as some non-polar and chlorinated pesticides are hard to ionize with an electrospray ion source.13,14
To show the expediency of a single method of analysis, the application team created an LC-MS/MS method using both ESI and APCI procedures to carry out an analysis of all the pesticides on the California regulated pesticide list.
This offered further advantages, including lower complexity, higher throughput and less expenditure on analysis.
The dirty matrix common in cannabis samples generally results in a build-up on the sampling interface of GC-MS/MS and LC-MS/MS systems. This can lead to higher maintenance expenditure, more downtime and a loss of productivity.
The LC-MS/MS method developed is less susceptible to contamination from the dirty cannabis matrix.
Detectability and Reproducibility
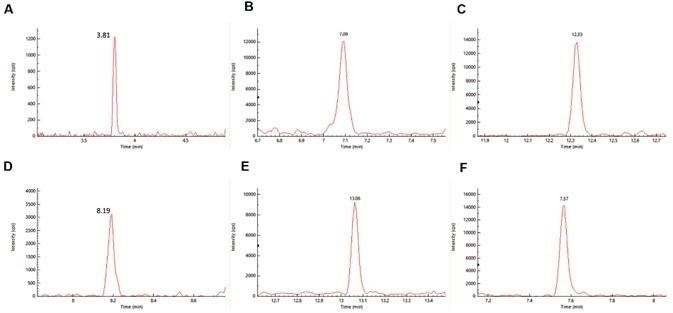
Figure 1. MRM chromatogram of representative set of pesticides-:(a) oxamyl, (b) metalaxyl, (c) fenpyroximate, (d) mycyclobutanil, (e) Etofenprox and (f) Azoxystrobin spiked at level of 0.01 μg/g in cannabis matrix. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
The MRM chromatograms with excellent signal to noise for a representative set of pesticides were found to have spiked at the low level of 0.01 µg/g in the cannabis flower (Figure 1).
Table 2. LOQs for California category II Pesticides with LC-MS/MS in Cannabis. Red/Green: Pesticides typically analyzed by GC-MS/MS, Red: Pesticides analyzed on LC-MS/MS by ESI, Green: Pesticides analyzed on Q-Sight by APCI. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S. No. |
Category II
Residual Pesticide |
LOQ |
Action
Level (μg/g) |
Action
Level/QSight
LOQ |
QSight
(μg/g) |
%CV
(n=7) |
| 1 |
Abamectin |
0.025 |
10.6 |
0.1 |
4 |
| 2 |
Acephate |
0.010 |
3.1 |
0.1 |
10 |
| 3 |
Acequinocyl |
0.025 |
13.3 |
0.1 |
10 |
| 4 |
Acetamiprid |
0.010 |
13.1 |
0.1 |
10 |
| 5 |
Azoxystrobin |
0.005 |
5.0 |
0.1 |
20 |
| 6 |
Bifenazate |
0.010 |
10.8 |
0.1 |
10 |
| 7 |
Bifenthrin |
0.010 |
14.4 |
0.5 |
50 |
| 8 |
Boscalid |
0.025 |
12.2 |
0.1 |
4 |
| 9 |
Captan |
0.25 |
7.0 |
0.7 |
2.8 |
| 10 |
Carbaryl |
0.010 |
9.5 |
0.5 |
50 |
| 11 |
Chlorantraniliprole |
0.025 |
5.6 |
10.0 |
400 |
| 12 |
Clofentezine |
0.010 |
11.3 |
0.1 |
10 |
| 13 |
Cyfluthrin |
0.25 |
19.1 |
1.0 |
4 |
| 14 |
Cypermethrin |
0.100 |
20.0 |
1.0 |
10 |
| 15 |
Diazinon |
0.005 |
3.8 |
0.2 |
40 |
| 16 |
Dimethomorph |
0.005 |
1.4 |
2.0 |
400 |
| 17 |
Etoxazole |
0.005 |
13.5 |
0.1 |
20 |
| 18 |
Fenhexamid |
0.010 |
12.5 |
0.1 |
10 |
| 19 |
Fenpyroximate |
0.005 |
6.9 |
0.1 |
20 |
| 20 |
Flonicamid |
0.010 |
10.2 |
0.1 |
10 |
| 21 |
Fludioxonil |
0.050 |
9.5 |
0.1 |
2 |
| 22 |
Hexythiazox |
0.005 |
8.4 |
0.1 |
20 |
| 23 |
Imidacloprid |
0.010 |
10.3 |
3.0 |
300 |
| 24 |
Kresoxim-methyl |
0.025 |
8.1 |
0.1 |
4 |
| 25 |
Malathion |
0.010 |
14.7 |
0.5 |
50 |
| 26 |
Metalaxyl |
0.010 |
8.0 |
2.0 |
200 |
| 27 |
Methomyl |
0.010 |
8.5 |
0.1 |
10 |
| 28 |
Myclobutanil |
0.010 |
10.4 |
0.1 |
10 |
| 29 |
Naled |
0.010 |
8.4 |
0.1 |
10 |
| 30 |
Oxamyl |
0.010 |
6.7 |
0.2 |
20 |
| 31 |
Pentachloronitrobenzene |
0.010 |
13.0 |
0.1 |
10 |
| 32 |
Permethrin |
0.010 |
16.0 |
0.5 |
50 |
| 33 |
Phosmet |
0.005 |
13.3 |
0.1 |
20 |
| 34 |
Piperonylbutoxide |
0.005 |
3.5 |
3.0 |
600 |
| 35 |
Prallethrin |
0.025 |
7.4 |
0.1 |
4 |
| 36 |
Propiconazole |
0.015 |
8.9 |
0.1 |
6.7 |
| 37 |
Pyrethrins |
0.1 |
1.4 |
0.5 |
5 |
| 38 |
Pyridaben |
0.010 |
7.9 |
0.1 |
10 |
| 39 |
Spinetoram |
0.005 |
13.8 |
0.1 |
20 |
| 40 |
Spinosad |
0.005 |
9.3 |
0.1 |
20 |
| 41 |
Spiromesifen |
0.010 |
9.4 |
0.1 |
10 |
| 42 |
Spirotetramat |
0.010 |
8.4 |
0.1 |
10 |
| 43 |
Tebuconazole |
0.005 |
11.0 |
0.1 |
20 |
| 44 |
Thiamethoxam |
0.010 |
3.6 |
4.5 |
450 |
| 45 |
Trifloxystrobin |
0.005 |
8.4 |
0.1 |
20 |
Table 3. LOQs for California Category II Mycotoxins with LC-MS/MS in Cannabis. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S. No. |
Category II
Mycotoxin |
LOQ |
Action
Level (μg/g) |
Action
Level/QSight
LOQ |
QSight
(μg/g) |
%CV
(n=7) |
| 1 |
Ochratoxin A |
0.010 |
18 |
0.020 |
2.0 |
| 2 |
Aflatoxin B1 |
0.001 |
18 |
NA |
NA |
| 3 |
Aflatoxin B2 |
0.0015 |
14 |
NA |
NA |
| 4 |
Aflatoxin G1 |
0.010 |
18 |
NA |
NA |
| 5 |
Aflatoxin G2 |
0.0015 |
19 |
NA |
NA |
| 6 |
Aflatoxin (B1+B2+G1+G2) |
0.005 |
NA |
0.020 |
4.0 |
Table 4. LOQs for California category I Pesticides with LC-MS/MS in cannabis. Red/Green : Pesticides typically analyzed by GC-MS/MS, Red: Pesticides Analyzed on LC-MS/MS by ESI Green: Pesticides Analyzed on LC-MS/MS by APCI. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S. No. |
Category I
Residual Pesticide |
LC-MS/MS LOQ |
Action
Level (μg/g) |
Action
Level/LOQ |
| (μg/g) |
%CV (n=7) |
| 1 |
Aldicarb |
0.010 |
10.6 |
0.1 |
10 |
| 2 |
Carbofuran |
0.010 |
3.1 |
0.1 |
10 |
| 3 |
Chlordane |
0.05 |
13.3 |
0.1 |
2 |
| 4 |
Chlorfenpyr |
0.05 |
6.0 |
0.1 |
2 |
| 5 |
Chlorpyrifos |
0.010 |
5.0 |
0.1 |
10 |
| 6 |
Coumaphos |
0.010 |
10.8 |
0.1 |
10 |
| 7 |
Daminozide |
0.015 |
14.4 |
0.1 |
6.67 |
| 8 |
DDVP (Dichlorvos) |
0.025 |
12.2 |
0.1 |
4 |
| 9 |
Dimethoate |
0.010 |
3.8 |
0.1 |
10 |
| 10 |
Ethoprophos |
0.010 |
9.5 |
0.1 |
10 |
| 11 |
Etofenprox |
0.010 |
5.6 |
0.1 |
10 |
| 12 |
Fenoxycarb |
0.010 |
11.3 |
0.1 |
10 |
| 13 |
Fipronil |
0.010 |
19.1 |
0.1 |
10 |
| 14 |
Imazalil |
0.010 |
23.1 |
0.1 |
10 |
| 15 |
Methiocarb |
0.010 |
3.8 |
0.1 |
10 |
| 16 |
Methyl parathion |
0.040 |
1.4 |
0.1 |
2.5 |
| 17 |
Mevinphos |
0.025 |
13.5 |
0.1 |
4 |
| 18 |
Paclobutrazol |
0.010 |
12.5 |
0.1 |
10 |
| 19 |
Propoxur |
0.010 |
6.9 |
0.1 |
10 |
| 20 |
Spiroxamine |
0.010 |
10.2 |
0.1 |
10 |
| 21 |
Thiacloprid |
0.010 |
9.5 |
0.1 |
10 |
LOQs (limits of quantification) and response reproducibility at LOQ level for each category 1 and 2 pesticide - and mycotoxins - in the cannabis extract are summarized in Tables 2, 3 and 4.
LOQs were established by considering the signals of the quantifier and qualifier ions (each S/N > 10), as well as ensuring the product ion ratios were inside the 20% tolerance limits of the expected ratio.
The LOQs determined in the study were comfortably under California’s action limit by factors in the range of 2 to 600 for all mycotoxins and category 2 pesticides listed – (Tables 2 and 3).
The response RSD for every pesticide and mycotoxin at its LOQ level in the cannabis matrix was under 20%.
The retention time for each analyte was reproducible within ± 0.1 minutes over a period of 24 hours, demonstrating that this method is sensitive and reproducible enough for mycotoxin and pesticide analysis in cannabis samples at the limit specified by California’s state regulations.
Sample matrix-matched calibration standards
Employment of matrix-matched calibration is the favored analytical process for quantitation, as it compensates for the matrix effects prevalent in cannabis samples.
An increased or decreased response is credited to ion suppression of the analytes during ionization caused by the presence of co-eluted matrix compounds.
The samples’ matrix effects required that a matrix-matched calibration curve was utilized for quantitation.
The curve was generated by injecting blank cannabis flower extract samples and blank cannabis flower extracts spiked with variable concentrations of mycotoxins and pesticides over a range of 0.1-1000 ng/ml.
Calibration curves for all mycotoxins and pesticides were linear with a calibration fit of R2 greater than 0.99 for every analyte.
Recovery Studies With Solvent Extraction
The QuEChERS extraction technique is a widespread process for the extraction of low levels of contaminants, for example, pesticides from vegetable and fruit matrices with above average water content.15
It incorporates the extraction of a wide range of pesticides and removal of organic acids, sugars and more compounds frequently found in vegetables and fruit.16,17,18,19,20
The QuEChERS extraction technique is not an appropriate procedure for use in polar pesticides (for example, daminozide) that are listed in the regulatory framework of California and other states.
As it is too polar to be extracted effectively with QuEChERS, daminozide stays in the aqueous phase. It does not partition into the organic solvent during the salting out step.
Daminozide recovery from a cannabis matrix utilizing QuEChERS extraction was reported to have been 10%.10 A standard cannabis matrix includes compounds that are hydrophobic, for example, terpenes and cannabinoids.
As a result, the QuEChERS extraction method does not remove the compounds that interfere with the matrix during the salting out step.
A number of groups have attempted to create an advanced QuEChERS method with d-SPE step which uses adsorbents such as PSA to remove the matrix from a cannabis extract.
Adding a d-SPE step to the QuEChERS method leads to this method becoming more laborious and expensive. It also results in low recovery levels for compounds like spioroxamine, ochratoxin A, spinosad, spirotetramat, among others.11,12
This low recovery level is caused by these compounds binding to the PSA adsorbent in the d-SPE step.
The deficiencies of this QuEChERS method for the extraction of pesticides from a cannabis matrix led the application team to utilize a simple acetonitrile-based solvent extraction method in the extraction process.
To provide confirmation of this method, fortified cannabis flower samples were utilized to determine mycotoxin and pesticide recovery, with samples being tested to confirm the absence of pesticides before the spiking step.
A total of five cannabis flower samples were spiked at two levels - low and high - of all mycotoxins (0.02 and 0.1 µg/g) and pesticides (0.1 and 1 µg/g) standard.
The levels were determined based on regulatory limits of mycotoxins and pesticides in cannabis defined by states, including California.
The absolute recoveries of all five mycotoxins and 66 pesticides and at two different levels were found to be within an acceptable range of 70-120 % - RSD was less than 20% for the five cannabis flower samples (Tables 5, 6 and 7).
In the case of two pesticides, recovery values were not reported at low spiked value as this was under the LOQ value.
LC-MS/MS Method With Optimum MRM Transitions for Challenging Analytes in Cannabis Matrices
Cannabis is a difficult matrix to test, which is heightened by low pesticide concentration levels. To guarantee maximum analytical confidence, multiple MRM transitions involving a range of pesticides with minimum matrix interference in the cannabis matrix were determined for low-level detection.
The insecticide acequinocyl can be easily ionized as a protonated molecular ion in a standard. However, the MRM transitions – which were based on protonated molecular ions in the cannabis matri - showed poor LOQ of 0.5 to 1 µg/g - about five to 10 times higher than its action limit for the state of California.
MRM transitions were therefore based on alternative modes of ionization, such as adduct formation, to reduce matrix interference and achieve LOQ of 0.025 µg/g. This was four times below action limits for acequinocyl in the cannabis matrix.
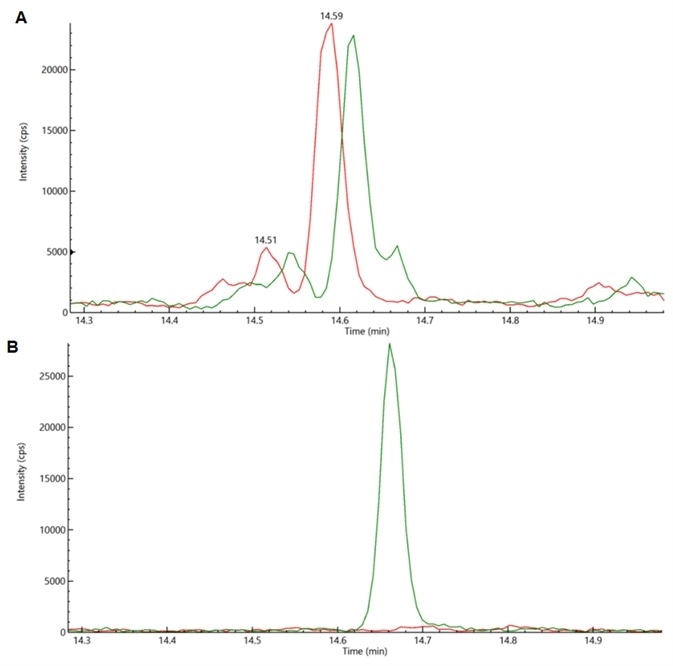
Figure 2. (a) Overlay of response of cannabis matrix (Red) and acequinocyl (Green) spiked at level of 0.1 μg/g in cannabis matrix with MRM transition based on protonated molecular ion and (b)Overlay of response of cannabis matrix (Red) and acequinocyl (Green) spiked at level of 0.1 μg/g in cannabis matrix with MRM transition based on adduct ion. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
The signal overlay of the blank cannabis matrix and acequinocyl spiked at a level of 0.1 µg/g in cannabis with MRM transitions based on protonated molecular ion and adduct ion of acequinocyl (Figure 2).
This result indicates that optimum acequinocyl MRM transitions facilitated achieving lower detection limits owing to minimal matrix interference.
High molecular weight compounds (for example, abamectin) and a number of early eluting polar compounds (such as daminozide) are difficult to measure at low levels using GC-MS/MS.
This is because these compounds decompose in a high-temperature GC injector or GC oven. It is possible to ionize high molecular weight compounds, such as abamectin, and polar compounds, such as daminozide, with the ESI source, but these also tend to decompose at high temperatures.
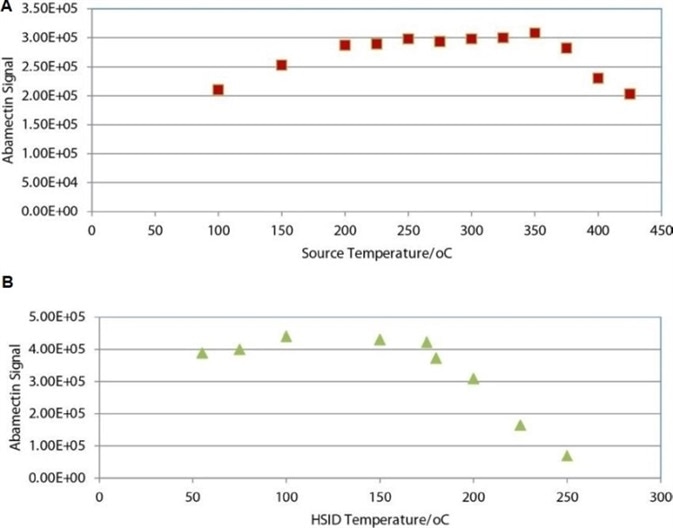
Figure 3. Abamectin signal as a function of ESI source (a) and HSID temperature (b). Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Figure 3 displays abamectin response as a function of HSID and source temperature. These results indicate that the optimum temperature values for the ESI source and HSID temperature were set to maximize signals for polar pesticides and high molecular weight abamectin.
These are prone to potassium and sodium adduct formation caused by potassium and sodium ions leached into the mobile phase from glassware.
The difficulties in controlling the quantity of potassium and sodium ions leached from glassware meant the utilization of the sodium adduct for abamectin as Q1 - the parent ion - mass for analysis would lead to response variation.
A controlled amount of ammonium salt was added to the mobile phase to reduce potassium or sodium adduct formation. Combining ammonium salt in the mobile phase with the best possible temperature conditions resulted in abamectin signals that were good and reproducible.
Table 5. Recovery of Category II pesticides at two different levels from cannabis with acetonitrile solvent extraction method. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S.No. |
Category II
Residual Pesticide |
Low Level 0.1 μg/g |
High Level 1 μg/g |
| Recovery% |
RSD % (n=5) |
Recovery % |
RS% (n=5) |
| 1 |
Abamectin |
85 |
10 |
89 |
9 |
| 2 |
Acephate |
93 |
16 |
91 |
9 |
| 3 |
Acequinocyl |
90 |
11 |
86 |
6 |
| 4 |
Acetamiprid |
87 |
13 |
95 |
9 |
| 5 |
Azoxystrobin |
87 |
12 |
92 |
8 |
| 6 |
Bifenazate |
88 |
8 |
88 |
7 |
| 7 |
Bifenthrin |
84 |
13 |
94 |
7 |
| 8 |
Boscalid |
87 |
10 |
89 |
5 |
| 9 |
Captan |
NA |
NA |
70 |
15 |
| 10 |
Carbaryl |
84 |
12 |
92 |
10 |
| 11 |
Chlorantraniliprole |
88 |
13 |
90 |
8 |
| 12 |
Clofentezine |
87 |
13 |
91 |
12 |
| 13 |
Cyfluthrin |
NA |
NA |
97 |
17 |
| 14 |
Cypermethrin |
98 |
18 |
85 |
13 |
| 15 |
Diazinon |
96 |
10 |
95 |
10 |
| 16 |
Dimethomorph |
87 |
15 |
90 |
7 |
| 17 |
Etoxazole |
89 |
10 |
92 |
10 |
| 18 |
Fenhexamid |
87 |
12 |
87 |
7 |
| 19 |
Fenpyroximate |
87 |
9 |
93 |
8 |
| 20 |
Flonicamid |
93 |
15 |
92 |
12 |
| 21 |
Fludioxonil |
94 |
13 |
93 |
8 |
| 22 |
Hexythiazox |
86 |
11 |
93 |
7 |
| 23 |
Imidacloprid |
89 |
11 |
91 |
9 |
| 24 |
Kresoxim-methyl |
91 |
10 |
95 |
8 |
| 25 |
Malathion |
90 |
12 |
91 |
7 |
| 26 |
Metalaxyl |
86 |
10 |
92 |
8 |
| 27 |
Methomyl |
89 |
10 |
90 |
9 |
| 28 |
Myclobutanil |
84 |
10 |
93 |
7 |
| 29 |
Naled |
87 |
10 |
91 |
7 |
| 30 |
Oxamyl |
93 |
16 |
94 |
9 |
| 31 |
Pentachloronitrobenzene |
80 |
16 |
88 |
8 |
| 32 |
Permethrin |
87 |
17 |
92 |
9 |
| 33 |
Phosmet |
86 |
11 |
91 |
7 |
| 34 |
Piperonylbutoxide |
91 |
8 |
94 |
8 |
| 35 |
Prallethrin |
88 |
15 |
94 |
8 |
| 36 |
Propiconazole |
90 |
14 |
95 |
11 |
| 37 |
Pyrethrins |
89 |
12 |
93 |
9 |
| 38 |
Pyridaben |
84 |
13 |
92 |
9 |
| 39 |
Spinetoram |
93 |
13 |
94 |
9 |
| 40 |
Spinosad |
88 |
14 |
90 |
10 |
| 41 |
Spiromesifen |
90 |
8 |
92 |
5 |
| 42 |
Spirotetramat |
97 |
10 |
90 |
7 |
| 43 |
Tebuconazole |
94 |
12 |
91 |
7 |
| 44 |
Thiamethoxam |
90 |
10 |
95 |
10 |
| 45 |
Trifloxystrobin |
86 |
12 |
93 |
9 |
Table 6. Recovery of Category II mycotoxins at two different levels from cannabis with acetonitrile solvent extraction method. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S.No. |
Category II
Mycotoxin |
Low Level 0.1 μg/g |
High Level 1 μg/g |
| Recovery% |
RSD % (n=5) |
Recovery % |
RS%(n=5) |
| 1 |
Aflatoxin 81 |
75 |
15 |
84 |
9 |
| 2 |
Aflatoxin 82 |
78 |
14 |
82 |
9 |
| 3 |
Aflatoxin G1 |
76 |
12 |
85 |
7 |
| 4 |
Aflatoxin G2 |
79 |
12 |
84 |
6 |
| 5 |
Ochratoxin A |
78 |
20 |
83 |
7 |
Table 7. Recovery of Category I pesticides at two different levels from cannabis with acetonitrile solvent extraction method. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S.No. |
Category I
Residual Pesticide |
Low Level 0.1 μg/g |
High Level 1 μg/g |
| Recovery% |
RSD % (n=5) |
Recovery % |
RS% (n=5) |
| 1 |
Aldicarb |
87 |
11 |
94 |
11 |
| 2 |
Carbofuran |
86 |
11 |
91 |
9 |
| 3 |
Chlordane |
87 |
19 |
92 |
10 |
| 4 |
Chlorfenapyr |
95 |
15 |
99 |
10 |
| 5 |
Chlorpyrifos |
94 |
8 |
92 |
8 |
| 6 |
Coumaphos |
90 |
12 |
95 |
10 |
| 7 |
Daminozide |
82 |
15 |
80 |
14 |
| 8 |
DDVP (Dichlorvos) |
94 |
14 |
91 |
11 |
| 9 |
Dimethoate |
89 |
11 |
96 |
9 |
| 10 |
Ethoprop(hos) |
92 |
9 |
94 |
7 |
| 11 |
Etofenprox |
88 |
13 |
93 |
8 |
| 12 |
F enoxycarb |
91 |
11 |
93 |
7 |
| 13 |
Fipronil |
89 |
9 |
95 |
8 |
| 14 |
Imazalil |
86 |
10 |
89 |
10 |
| 15 |
Methiocarb |
81 |
9 |
93 |
6 |
| 16 |
Methyl parathion |
89 |
14 |
96 |
11 |
| 17 |
Mevinphos |
86 |
10 |
95 |
10 |
| 18 |
Paclobutrazol |
79 |
13 |
90 |
6 |
| 19 |
Propoxur |
91 |
13 |
93 |
9 |
| 20 |
Spiroxamine |
88 |
9 |
89 |
9 |
| 21 |
Thiacloprid |
89 |
13 |
95 |
10 |
Analysis of Pesticides Typically Analyzed By GC-MS/MS and LC-MS/MS
A variety of pesticides in cannabis, regulated by California and other states, are traditionally analyzed using GC-MS/MS with an EI source since these pesticides have low proton affinity.
This low proton affinity results in low ionization efficiency with the ESI source. Pesticides typically analyzed utilizing GC/MS include cypermethrin, captan, cyfluthrin, permethrin, naled and pyrethrins.
The required sensitivity was achieved by optimizing the selected MRMs with a heated electrospray source. LOQ for these analytes was found to be well below the California action limits, in the range of 0.01 to 0.25 µg/g.
Analysis of Pyrethrin Isomers in Cannabis
Pyrethrins are a class of organic compounds usually derived from chrysanthemum cinerariifolium. These compounds possess potent insecticidal activity due to the way they target insects’ nervous systems.
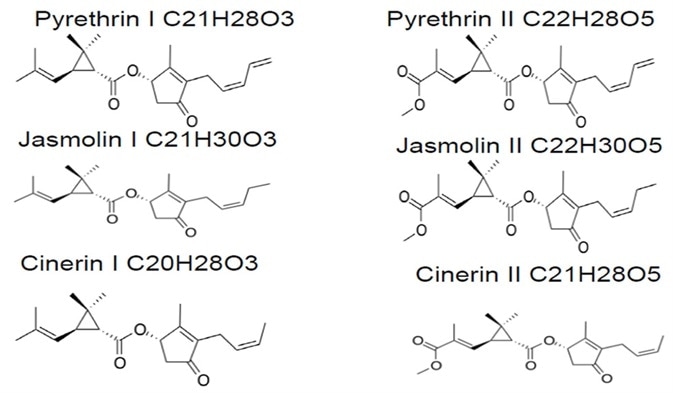
Figure 4. Structure of 6 isomers of pyrethrins. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Figure 4 illustrates pyrethrins (a group of six isomers) and their structures.
Naturally occurring pyrethrins are extracted from chrysanthemum flowers. These are esters of pyrethric acid (for example, pyrethrin II, cinerin II and jasmolin II) and esters of chrysanthemic acid (for example, pyrethrin I, cinerin I and jasmolin I).
The United States specifies that pyrethrum extract should be standardized at 45–55% w/w total pyrethrins. In a commercially available pyrethrin standard, the percentage of pyrethins are approximately as follows:
- Pyrethrin I - 56.1%
- Pyrethrin II - 27.8%
- Cinerin I - 5.7%
- Cinerin II - 3.8%
- Jasmolin I - 4%
- Jasmolin II - 2.6%
Several compounds in cannabis can mimic pyrethrins’ structure, and the analysis of pyrethrins in cannabis can be further complicated due to matrix interference.
Optimal MRM transitions and LC gradients were developed to analyze the six isomers of pyrethrins at low levels in the cannabis matrix with a minimum of matrix interference.
The LC-MS/MS method utilizing optimum MRM transitions and LC gradient gave the following LOQs for the six pyrethrins in cannabis flowers:
- Pyrethrin I - 0.1 µg/g
- Pyrethrin II - 0.1 µg/g
- Cinerin I - 0.01 µg/g
- Cinerin II - 0.03 µg/g
- Jasmolin I - 0.025 µg/g
- Jasmolin II - 0.01 µg/g
APCI Analysis of Pesticides That Do Not Ionize Effectively With ESI
Hydrophobic and halogenated pesticides such as chlordane and pentachloronitrobenzene are usually analyzed using GC-MS/MS as they do not ionize effectively utilizing LC-MS/MS with an ESI source (Figure 5).
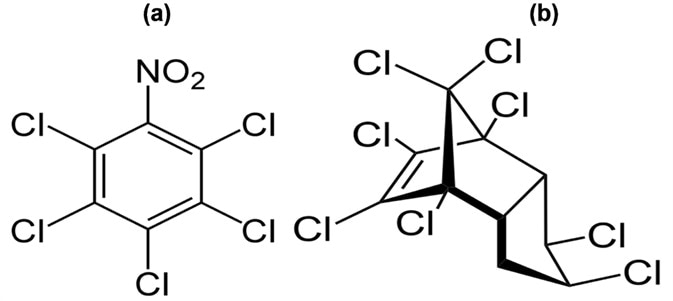
Figure 5. Structure of pentachloronitrobenzene (a) and chlordane (b). Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
As pentachloronitrobenzene (PCNB) does not contain hydrogen atoms, for loss of protons, or functional groups with either high proton affinity or which can form sodium or ammonia or adducts, it cannot be ionized with the ESI source.
Chlordane is highly chlorinated and possesses a very low proton affinity, making it hard to ionize effectively with an ESI source.
An APCI ion source is more suited for ionization of very hydrophobic and non-polar analytes. The APCI was utilized to establish the detection limits of chlordane and pentachloronitrobenzene in cannabis.
The APCI ion source was also utilized for low-level analysis of chlorfenapyr in cannabis as detection limits for chlorfenapyr were improved by a factor of two with an APCI source when compared to an ESI source due to lower ion suppression.
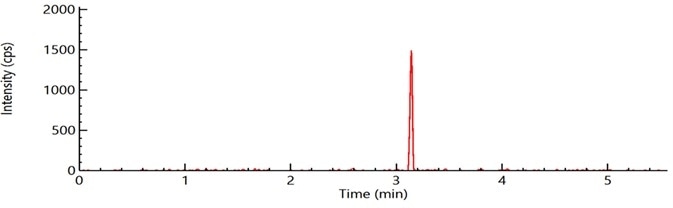
Figure 6. Sample chromatogram of pentachloronitrobenzene (PCNB) spiked at level of 0.1 μg/g in a cannabis matrix using LC-MS/MS system with APCI source. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Excellent signal to noise results (S/N >= 100) for pentachloronitrobenzene spiked at a level of 0.1 µg/g in the cannabis matrix employing an LC-MS/MS system with an APCI source (Figure 6).
Utilizing a high-speed, 6-minute LC-MS/MS method with a short LC gradient and an APCI source, LOQs in cannabis were as follows:
- Pentachloronitrobenzene - 0.01 µg/g
- Chlordane - 0.05 µg/g
- Chlorfenapyr - 0.05 µg/g
Long-Term Stability Data With StayClean™ Source in LC-MS/MS
Long-term stability data for mycotoxin and pesticide analysis in cannabis samples were collected using a LC-MS/MS system, which was fitted with dual APCI and ESI sources.
The LC-MS/MS system was combined with a heated and self-cleaning StayClean™ source with a laminar flow interface.
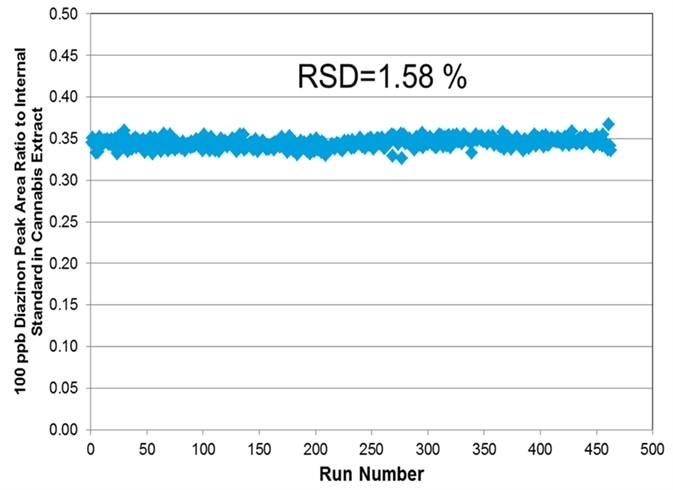
Figure 7. Long term stability data over one week of injections of diazinon at a level of 100 ng/mL spiked in cannabis flower matrix extract. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
The long-term stability and response of this method for 100 ng/ml of diazinon spiked in cannabis extract over seven days can be seen in Figure 7.
This indicates that pesticide analysis in cannabis showed that response RSD over that time period for the majority of mycotoxins and pesticides ranged from 1.5-20%.
The results indicated that a heated self-cleaning source in an LC-MS/MS system would reduce the maintenance requirements common with this matrix.
The majority of published LC-MS/MS methods do not display long-term stability data. They also highlight the need to clean the electrospray source regularly to preserve the sensitivity of the mass spectrometer.21
Published studies also divert the LC flow to waste for the first few minutes and after the final peak elutes out. This is done to reduce contamination from unretained and late eluting matrix compounds.
The examples presented here confirm that excellent long-term stability data was acquired without diverting the LC flow from the MS in the first few minutes and at the end of a run. There were also no requirements for periodic ion source cleaning.
Conclusions
This study demonstrated that the presented LC-MS/MS method for analysis of different mycotoxin and pesticide residues in cannabis samples was unique, rapid, quantitative and reliable.
The solvent extraction method outlined above is appropriate for labs needing to conform to California’s state regulations due to the recovery of all mycotoxins and pesticides from a cannabis matrix being in the acceptable range of 70-120% and with RSD less than 20%.
The method enabled the precise identification and quantification of all five mycotoxins and all 66 pesticides at low levels - 0.005 to 0.25 µg/g - well below the action limits set out by the state of California.
The capacity to both screen and quantitate all 66 pesticides (including the very hydrophobic and chlorinated compounds generally analyzed on a GC-MS/MS), as well as the five mycotoxins, proves that this method is a unique process for screening and quantifying mycotoxins and pesticides in cannabis samples with a single instrument.
References
- A.A. Monte, R.D. Zane and K. J. Heard, JAMA, 313(3), 241-242 (2015).
- J. C. Raber, S. Eizinga and C. Kaplan, J. Toxicol. Sci., 40(6), 797-803 (2015).
- D. Stone, Regul. Toxicol. Pharmacol., 69(3), 284-288 (2014).
- Elise Mcdonough, “Tainted: The Problem With Pot and Pesticides,” High times, 2017, available from https://hightimes. com/grow/tainted-the-problem-with-pot-and-pesticides.
- Alicia Lozano, “Pesticides in Marijuana Pose a Growing Problem for Cannabis Consumers,” LA Weekly, 2016, available from https://www.laweekly.com/.
- http://american-safe-access.s3.amazonaws.com/documents/ AHP_ Cannabis_Monograph_Preview.pdf
- https://www.aocs.org/.
- Exhibit A, Table 3. Pesticide analytes and their action levels. Oregon Administrative Rules 333-007-0400; Oregon/gov/ oha, effective 5/31/2017.
- Chapter 5. Testing Laboratories Section 5313 Residual Pesticides, Bureau of Marijuana Control Proposed Text of Regulations, CA Code of Regulations, Title 16, 42, pp 23-26.
- K. K. Stenerson and G. Oden, Cann. Sci. and Tech., 1(1), 48-53 (2018).
- J. Kowlaski, J. H. Dahl, A. Rigdon, J. Cochran, D. Laine and G. Fagras, LCGC, 35(5) 8-22 (2017).
- X. Wang, D. Mackowsky, J. Searfoss and M. Telepchak, LCGC, 34(10), 20-27 (2016).
- L. Alder, K. Greulich, G. Kempe and B. Vieth, Mass. Spec. Rev., 25, 838– 865 (2006).
- United States Department of Agriculture Food Safety and Inspection Service, Office of Public Health Science,” Screening for Pesticides by LC/MS/MS and GC/MS/MS,” 2018, available from https://www.fsis.usda.gov/wps/wcm/connect/499a8e9e- 49bd-480a-b8b6-d1867f96c39d/CLG-PST5. pdf?MOD=AJPERES.
- M. Anastassiades, S. J. Lehotay, D. Stajnbaher and F.J. Schenk, J. AOAC Int., 86(2), 412-431 (2003).
- S. W. C. Chung and B.T. P. Chan, J. Chromatogr. A, 1217, 4815-4824 (2010).
- S.C. Cunha, S. J. Lehotay, K. Mastovska, J. O. Fernandes, M. Beatriz and P. P. Oliveria, J. Sep. Sci., 30(4), 620-626 (2007).
- Y. Sapozhinikova, J. Agric. Food Chem., 62, 3684-3689 (2014).
- J. Wang and W. Cheung, J. AOAC Int. 99(2), 539-557 (2016).
- M. Villar-Pulido, B. Gilbert-Lopez, J. F. Garcia Reyes, N. R. Martos, and A. Molina-Diaz, Talanta 85, 1419-1427 (2011).
- L. Geis-Asteggiante, S. J. Lehotay, R. A. Lightfield, T. Dutko, C. Ng and L. Bluhm, J. Chromatogr. A, 1258, 43-54 (2012).
Acknowledgments
Produced from materials originally authored by Avinash Dalmia, Erasmus Cudjoe, Toby Astill, Jacob Jalali, Jason P Weisenseel, and Feng Qin from PerkinElmer; Molly Murphy and Travis Ruthenberg from SC Labs.
About PerkinElmer Cannabis & Hemp Testing Solutions
 With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
They help drive analytical best practices and operating procedures and commit to ensuring your laboratory has maximum uptime. Learn about their various instruments, testing methods, and applications for cannabis analyses. Let them work with you to build an efficient workflow, so you can focus on growing your business.
Sponsored Content Policy: AZO Life Science publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZO Life Science, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.