The recreational use of cannabis was legalized in Canada in the fall of 2018. This new market required the development and implementation of stringent domestic and international cannabis safety and quality control regulations.
Health Canada recently introduced a series of regulations that required mandatory pesticide testing of all cannabis products.
These regulations require Canadian licensed producers (LPs) to deliver representative cannabis product samples to independent laboratories prior to sale in order for products to be screened and quantified for pesticide content.
The growing need for credible, accurate data has seen high-performance liquid chromatography-tandem mass spectrometry (LC/MS/MS) emerge as the most preferred means of pesticide analysis.
LC/MS/MS offers superior sensitivity, selectivity and ruggedness while requiring minimal pre-analysis sample preparation.
Gas chromatography-mass spectrometry (GC/MS/MS) methods are available for the analysis of pesticides in cannabis samples, though these are only applicable to a limited subset of analytes.
A number of compounds (for example, the high molecular weight compound abamectin) are not suitable for analysis via GC/MS/MS due to heat lability and a tendency to degrade in the GC injection port or in the GC column when exposed to high temperatures.
GC/MS/MS methods are less robust than LC/MS/MS methods when performing pesticide analysis in complex matrices due to their need for extensive sample preparation in order to avoid GC injection port contamination.1,2
While the US does not provide federal guidance on the analysis of pesticide analytes in cannabis samples, Canada has established thorough guidelines for pesticide residue analysis in cannabis, including regulatory limits for 96 pesticides.
Neither country, however, has federal method guidelines in place, meaning that laboratories are responsible for developing and validating their own approaches to pesticide testing.
A number of reports have been published on pesticide analysis in cannabis, but these studies are lacking in some areas.3,4,5 The majority of these studies either fail to achieve detection limits in line with Canadian action limits, or they employ time-consuming sample preparation methods such as QuEChERS with dSPE.
These sample preparation methods not only demonstrate poor recoveries for some pesticides, but they also necessitate the use of both LC/MS/MS and GC/MS/MS techniques to properly analyze the full range of regulated pesticides.
The use of these processes can significantly increase analysis complexity, cost, uncertainty and turnaround time.
In the examples presented here, all 96 pesticides were analyzed in cannabis flower extracts, including highly hydrophobic and chlorinated pesticides generally analyzed by GC/MS/MS.
An LC/MS/MS instrument featuring dual source ESI and APCI modes was employed in order to meet the required low regulatory limits set by Health Canada for all 96 pesticides. A basic solvent extraction method was used. This led to recoveries in the acceptable range of 70-120% for all analytes.
Experimental
Hardware and Software
A PerkinElmer QSight® LX50 UHPLC system was used for chromatographic separation. Detection was achieved via a PerkinElmer QSight 420 MS/MS detector utilizing a dual ionization ESI and APCI source. These sources operate independently and maintain two separate inlets.
The Simplicity™ 3Q software platform was utilized for instrument control, as well as data acquisition and processing.
Sample Preparation Method
The sample preparation procedure is outlined below. This was performed step-by-step with 20-fold dilution:
- Approximately 5 g of cannabis flower was taken as a representative sample of each batch. This was ground finely using a grinder.
- Exactly 1 g of sample was weighed and placed into a 50 mL centrifuge tube.
- The sample was then spiked with 10 µL of internal standard solution.
- Three steel balls (10 mm in diameter) were added to the tube to facilitate efficient extraction during vortex mixing.
- A total of 10 mL of LC/MS grade acetonitrile was added to the tube before this was capped.
- The tube was placed on a multi-tube vortex mixer and allowed to vortex for 10 minutes.
- The extract in the tube was centrifuged for 10 minutes at 3000 rpm.
- The solvent was filtered into a 5 mL glass amber vial using a 0.22 micron nylon syringe filter. This was then capped.
- The bottle was then labeled with the sample ID.
- A total of 0.5 mL of extracted sample was transferred into a 2 mL HPLC vial and diluted with 0.5 mL of LC/MS grade acetonitrile before being mixed.
LC Method and MS Source Conditions
Table 1 shows the complete set of LC method and MS source parameters.
Table 1. LC Method and MS Source Conditions. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| LC Conditions |
| LC Column |
PerkinElmer Quasar™ SPP Pesticides (4.6 × 100 mm, 2.7 µm) |
Mobile Phase A
(ESI Method) |
2 mM ammonium formate + 0.1% formic acid (in water) |
Mobile Phase B
(ESI Method) |
2 mM ammonium formate + 0.1% formic acid (in methanol) |
Mobile Phase A
(APCI Method) |
LC/MS grade water |
Mobile Phase B
(APCI Method) |
LC/MS grade methanol |
| Mobile Phase Gradient |
The run time for the optimized gradient elution method, including analytical column re-conditioning, was 18.5 minutes for the ESI method, and 6 minutes. for APCI. The final method ensured separation of the bulk cannabis matrix from the analytes for improved quantitation. |
| Column Oven Temperature |
30 ºC |
| Auto sampler Temperature |
20 ºC |
| Injection Volume |
3.0 µL for LC/MS/MS method with ESI source. 10 µL for LC/MS/MS method with APCI source. |
| MS Source Conditions for ESI Source and APCI Source |
| ESI Voltage (Positive) |
+5100 V |
| ESI Voltage (Negative) |
-4200 V |
| APCI Corona Discharge |
-3 µA |
| Drying Gas |
150 arbitrary units |
| Nebulizer Gas |
350 arbitrary units |
| Source Temperature (ESI Method) |
315 ºC |
| Source Temperature (APCI Method) |
275 ºC |
| HSID Temperature (ESI Method) |
200 ºC |
| HSID Temperature (APCI Method) |
200 ºC |
| Detection Mode |
Time-managed MRM™ |
Results and Discussion
Detectability and Reproducibility
Many laboratories employ numerous analytical instruments and laborious sample preparation methods in order to meet the low pesticide limits required by Health Canada.
An LC/MS/MS method is presented here that is amenable to in-lab validation. This method utilizes a PerkinElmer liquid chromatograph in conjunction with the tandem mass spectrometer (QSight LX50 and mass spectrometer).
This innovative combination is able to achieve full analysis of the 96 pesticides outlined in the Health Canada regulations.
In the examples presented here, all 96 pesticides were analyzed using the QSight 420 dual source mass spectrometer equipped with both APCI and ESI ionization probes.
The single platform PerkinElmer LC/MS/MS system was able to detect a range of pesticides typically analyzed via gas chromatography; for example, kinoprene, methoprene, methyl parathion, etridiazole, endosulfans, cypermethrin, cyfluthrin and quintozene.
Table 2 summarizes the limits of quantification (LOQs) and response reproducibility at the LOQ level for each pesticide present in the cannabis extract.
Table 2. LOQs for Health Canada Regulated Pesticides with LC/MS/MS in Cannabis. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S. No. |
Pesticide |
LOQ |
Action Level (µg/g) |
Action Level/LOQ |
| LC/MS/MS (µg/g) |
%CV (n=3) |
| 1 |
Abamectin |
0.1 |
8.2 |
0.1 |
1 |
| 2 |
Acephate |
0.01 |
4.8 |
0.02 |
2 |
| 3 |
Acetamiprid |
0.02 |
2.8 |
0.1 |
5 |
| 4 |
Acequinocyl |
0.01 |
6.5 |
0.03 |
3 |
| 5 |
Aldicarb |
0.01 |
8.6 |
1 |
100 |
| 6 |
Allethrin |
0.05 |
3.5 |
0.2 |
4 |
| 7 |
Azadirachtin |
0.1 |
18.6 |
1 |
10 |
| 8 |
Azoxystrobin |
0.0025 |
12.2 |
0.02 |
8 |
| 9 |
Benzovindiflupyr |
0.01 |
14.5 |
0.02 |
2 |
| 10 |
Bifenazate |
0.0025 |
4.8 |
0.02 |
8 |
| 11 |
Bifenthrin |
0.005 |
18.6 |
1 |
200 |
| 12 |
Boscalid |
0.01 |
8.0 |
0.02 |
2 |
| 13 |
Buprofezin |
0.0025 |
3.1 |
0.02 |
8 |
| 14 |
Carbaryl |
0.01 |
9.1 |
0.05 |
5 |
| 15 |
Carbofuran |
0.01 |
5.1 |
0.02 |
2 |
| 16 |
Chlorantraniliprole |
0.01 |
6.2 |
0.02 |
2 |
| 17 |
Chlorphenapyr |
0.02 |
3.3 |
0.05 |
2.5 |
| 18 |
Chlorpyrifos |
0.01 |
8.5 |
0.04 |
4 |
| 19 |
Clofentezine |
0.01 |
6.9 |
0.02 |
2 |
| 20 |
Clothianidin |
0.02 |
10.6 |
0.05 |
2.5 |
| 21 |
Coumaphos |
0.01 |
9.9 |
0.02 |
2 |
| 22 |
Cyantranilipole |
0.01 |
4.8 |
0.02 |
2 |
| 23 |
Cyfluthrin |
0.2 |
15.8 |
0.2 |
1 |
| 24 |
Cypermethrin |
0.2 |
11.3 |
0.3 |
1.5 |
| 25 |
Cyprodinil |
0.01 |
14.0 |
0.25 |
25 |
| 26 |
Daminozide |
0.02 |
1.7 |
0.1 |
5 |
| 27 |
Deltamethrin |
0.1 |
11.1 |
0.5 |
5 |
| 28 |
Diazinon |
0.01 |
8.5 |
0.02 |
2 |
| 29 |
Dichlorvos |
0.01 |
10.2 |
0.1 |
10 |
| 30 |
Dimethoate |
0.0025 |
5.9 |
0.02 |
8 |
| 31 |
Dimethomorph |
0.01 |
5.6 |
0.05 |
5 |
| 32 |
Dinotefuran |
0.0025 |
7.9 |
0.1 |
40 |
| 33 |
Dodemorph |
0.01 |
13.0 |
0.05 |
5 |
| 34 |
Endosulfan-alpha |
0.1 |
16.2 |
0.2 |
2 |
| 35 |
Endosulfan-beta |
0.05 |
10.2 |
0.05 |
1 |
| 36 |
Endosulfan sulfate |
0.01 |
12.3 |
0.05 |
5 |
| 37 |
Ethoprophos |
0.01 |
12.4 |
0.02 |
2 |
| 38 |
Etofenprox |
0.0025 |
9.8 |
0.05 |
20 |
| 39 |
Etoxazole |
0.01 |
13.0 |
0.02 |
2 |
| 40 |
Etridiazole |
0.02 |
18.4 |
0.03 |
1.5 |
| 41 |
Fenoxycarb |
0.01 |
1.8 |
0.02 |
2 |
| 42 |
Fenpyroximate |
0.0025 |
12.4 |
0.02 |
8 |
| 43 |
Fensulfothion |
0.0025 |
5.1 |
0.02 |
8 |
| 44 |
Fenthion |
0.01 |
3.2 |
0.02 |
2 |
| 45 |
Fenvalerate |
0.02 |
14.7 |
0.1 |
5 |
| 46 |
Fipronil |
0.01 |
4.6 |
0.06 |
6 |
| 47 |
Flonicamid |
0.01 |
7.0 |
0.05 |
5 |
| 48 |
Fludioxonil |
0.01 |
7.4 |
0.02 |
2 |
| 49 |
Fluopyram |
0.0025 |
9.3 |
0.02 |
8 |
| 50 |
Hexythiazox |
0.0025 |
7.4 |
0.01 |
4 |
| 51 |
Imazalil |
0.02 |
6.0 |
0.05 |
2.5 |
| 52 |
Imidacloprid |
0.01 |
4.8 |
0.02 |
2 |
| 53 |
Iprodione |
0.02 |
4.5 |
1 |
50 |
| 54 |
Kinoprene |
0.2 |
8.8 |
0.5 |
2.5 |
| 55 |
Kresoxim-methyl |
0.01 |
6.5 |
0.02 |
2 |
| 56 |
Malathion |
0.0025 |
11.0 |
0.02 |
8 |
| 57 |
Metalaxyl |
0.0025 |
5.8 |
0.02 |
8 |
| 58 |
Methiocarb |
0.0025 |
4.5 |
0.02 |
8 |
| 59 |
Methomyl |
0.01 |
5.7 |
0.05 |
5 |
| 60 |
Methoprene |
0.5 |
9.0 |
2 |
4 |
| 61 |
Methyl parathion |
0.02 |
9.4 |
0.05 |
2.5 |
| 62 |
Mevinphos |
0.01 |
3.7 |
0.05 |
5 |
| 63 |
MGK-264 |
0.01 |
2.7 |
0.05 |
5 |
| 64 |
Myclobutanil |
0.01 |
6.0 |
0.02 |
2 |
| 65 |
Naled |
0.01 |
8.2 |
0.1 |
10 |
| 66 |
Novaluron |
0.01 |
4.1 |
0.05 |
5 |
| 67 |
Oxamyl |
0.01 |
2.5 |
3 |
300 |
| 68 |
Paclobutrazol |
0.01 |
9.4 |
0.02 |
2 |
| 69 |
Permethrin |
0.01 |
10.9 |
0.5 |
50 |
| 70 |
Phenothrin |
0.05 |
5.1 |
0.05 |
1 |
| 71 |
Phosmet |
0.01 |
8.3 |
0.02 |
2 |
| 72 |
Piperonyl butoxide |
0.01 |
6.2 |
0.2 |
20 |
| 73 |
Pirimicarb |
0.01 |
4.3 |
0.02 |
2 |
| 74 |
Prallethrin |
0.01 |
9.1 |
0.05 |
5 |
| 75 |
Propiconazole |
0.04 |
5.7 |
0.1 |
2.5 |
| 76 |
Propoxur |
0.01 |
11.1 |
0.02 |
2 |
| 77 |
Pyraclostrobin |
0.01 |
6.2 |
0.02 |
2 |
| 78 |
Pyrethrins |
0.025 |
5.7 |
0.05 |
2 |
| 79 |
Pyridaben |
0.0025 |
1.7 |
0.05 |
20 |
| 80 |
Quintozene |
0.01 |
26.8 |
0.02 |
2 |
| 81 |
Resmethrin |
0.1 |
7.2 |
0.1 |
1 |
| 82 |
Spinetoram |
0.01 |
8.5 |
0.02 |
2 |
| 83 |
Spinosad |
0.01 |
12.6 |
0.1 |
10 |
| 84 |
Spirodiclofen |
0.02 |
12.7 |
0.25 |
12.5 |
| 85 |
Spiromesifen |
0.01 |
5.7 |
3 |
300 |
| 86 |
Spirotetramat |
0.01 |
9.8 |
0.02 |
2 |
| 87 |
Spiroxamine |
0.01 |
8.3 |
0.1 |
10 |
| 88 |
Tebuconazole |
0.01 |
10.9 |
0.05 |
5 |
| 89 |
Tebufenozide |
0.01 |
6.6 |
0.02 |
2 |
| 90 |
Teflubenzuron |
0.01 |
11.5 |
0.05 |
5 |
| 91 |
Tetrachlorvinphos |
0.01 |
6.9 |
0.02 |
2 |
| 92 |
Tetramethrin |
0.05 |
7.3 |
0.1 |
2 |
| 93 |
Thiacloprid |
0.01 |
4.7 |
0.02 |
2 |
| 94 |
Thiamethoxam |
0.0025 |
3.0 |
0.02 |
8 |
| 95 |
Thiophanate-methyl |
0.01 |
7.3 |
0.05 |
5 |
| 96 |
Trifloxystrobin |
0.0025 |
6.4 |
0.02 |
8 |
Note: Pesticides typically analyzed by GC/MS/MS are highlighted in red/green. Pesticides analyzed by ESI are highlighted in black or red. Pesticides Analyzed by APCI are highlighted in green.
LOQs were established by evaluating signals of the quantifier and qualifier ions (S/N > 10 for both) while confirming that product ion ratios remained within the 30% tolerance windows of the projected ratio.
Response relative standard deviation (RSD) for each pesticide at its LOQ level was found to be less than 30%. The retention time for each individual analyte was reproducible within ± 0.1 of a minute over a 24-hour period.
This confirms that the method is sensitive and reproducible enough to be used for pesticide analysis in cannabis.
Recovery studies with solvent extraction
Sample preparation is an essential stage in the analysis of pesticides in cannabis matrices.
Solvent extraction offers a rapid, straightforward means of achieving high extraction recovery when compared to time-consuming sample preparation techniques requiring larger sample volumes and multiple steps; for example, solid phase extraction (SPE) and QuEChERS.
This study, therefore, opted to use a solvent extraction method for the extraction of pesticides in cannabis.
To confidently confirm this method, fortified cannabis flower samples were utilized in the determination of pesticide recovery. Tests were performed to confirm the absence of pesticides before the cannabis flowers were spiked.
A total of three cannabis flower samples were spiked at two levels (0.02 and 0.2 μg/g). Every pesticide compound in the standard was used.
Table 3 illustrates the way in which absolute recoveries of all 96 pesticides at both spiked levels were found to be within an acceptable range of 70-120%. RSD was found to be less than 20% for each of the three cannabis flower samples.
Table 3. Recovery of all pesticides at two different levels with recommended sample preparation. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| S. No. |
Canada Pesticide |
Low Level 0.02 µg/g |
High Level 0.2 µg/g |
| Recovery/% |
RSD/% (n=3) |
Recovery/% |
RSD/% (n=3) |
| 1 |
Abamectin* |
100 |
10 |
100 |
8 |
| 2 |
Acephate |
103 |
2 |
102 |
9 |
| 3 |
Acetamiprid |
94 |
2 |
96 |
2 |
| 4 |
Acequinocyl* |
88 |
3 |
78 |
3 |
| 5 |
Aldicarb |
86 |
4 |
90 |
2 |
| 6 |
Allethrin* |
74 |
10 |
99 |
3 |
| 7 |
Azadirachtin* |
108 |
17 |
90 |
5 |
| 8 |
Azoxystrobin |
88 |
17 |
89 |
7 |
| 9 |
Benzovindiflupyr |
119 |
9 |
87 |
3 |
| 10 |
Bifenazate |
91 |
10 |
90 |
4 |
| 11 |
Bifenthrin |
95 |
3 |
85 |
3 |
| 12 |
Boscalid |
88 |
10 |
83 |
5 |
| 13 |
Buprofezin |
101 |
3 |
76 |
7 |
| 14 |
Carbaryl |
93 |
2 |
95 |
4 |
| 15 |
Carbofuran |
94 |
6 |
94 |
3 |
| 16 |
Chlorantraniliprole |
94 |
7 |
98 |
12 |
| 17 |
Chlorphenapyr |
92 |
6 |
85 |
6 |
| 18 |
Chlorpyrifos |
101 |
13 |
88 |
2 |
| 19 |
Clofentezine |
89 |
19 |
79 |
11 |
| 20 |
Clothianidin |
92 |
4 |
92 |
5 |
| 21 |
Coumaphos |
109 |
8 |
80 |
8 |
| 22 |
Cyantranilipole |
93 |
3 |
79 |
5 |
| 23 |
Cyfluthrin* |
- |
- |
100 |
12 |
| 24 |
Cypermethrin* |
- |
- |
103 |
8 |
| 25 |
Cyprodinil |
77 |
15 |
105 |
17 |
| 26 |
Daminozide* |
107 |
3 |
94 |
1 |
| 27 |
Deltamethrin |
117 |
-20 |
81 |
7 |
| 28 |
Diazinon |
93 |
9 |
79 |
5 |
| 29 |
Dichlorvos |
73 |
4 |
72 |
1 |
| 30 |
Dimethoate |
99 |
5 |
94 |
1 |
| 31 |
Dimethomorph |
98 |
10 |
94 |
1 |
| 32 |
Dinotefuran |
97 |
3 |
94 |
2 |
| 33 |
Dodemorph |
82 |
7 |
77 |
4 |
| 34 |
Endosulfan-alpha* |
83 |
6 |
84 |
2 |
| 35 |
Endosulfan-beta* |
91 |
7 |
94 |
3 |
| 36 |
Endosulfan sulfate |
92 |
4 |
94 |
6 |
| 37 |
Ethoprophos |
108 |
6 |
87 |
6 |
| 38 |
Etofenprox |
91 |
5 |
88 |
5 |
| 39 |
Etoxazole |
91 |
13 |
92 |
5 |
| 40 |
Etridiazol* |
75 |
9 |
75 |
7 |
| 41 |
Fenoxycarb |
85 |
14 |
81 |
7 |
| 42 |
Fenpyroximate |
117 |
9 |
100 |
4 |
| 43 |
Fensulfothion |
99 |
7 |
84 |
5 |
| 44 |
Fenthion |
89 |
19 |
88 |
7 |
| 45 |
Fenvalerate* |
92 |
12 |
98 |
9 |
| 46 |
Fipronil |
118 |
12 |
103 |
2 |
| 47 |
Flonicamid |
102 |
4 |
103 |
4 |
| 48 |
Fludioxonil |
104 |
3 |
103 |
8 |
| 49 |
Fluopyram |
90 |
7 |
80 |
3 |
| 50 |
Hexythiazox |
86 |
9 |
102 |
3 |
| 51 |
Imazalil |
104 |
11 |
87 |
3 |
| 52 |
Imidacloprid |
95 |
5 |
96 |
5 |
| 53 |
Iprodione* |
80 |
6 |
74 |
3 |
| 54 |
Kinoprene* |
- |
- |
106 |
11 |
| 55 |
Kresoxim-methyl |
93 |
10 |
93 |
7 |
| 56 |
Malathion |
88 |
5 |
96 |
5 |
| 57 |
Metalaxyl |
100 |
9 |
86 |
7 |
| 58 |
Methiocarb |
101 |
3 |
86 |
3 |
| 59 |
Methomyl |
93 |
12 |
93 |
5 |
| 60 |
Methoprene* |
- |
- |
110 |
8 |
| 61 |
Methyl parathion |
91 |
10 |
82 |
13 |
| 62 |
Mevinphos |
94 |
2 |
98 |
4 |
| 63 |
MGK-264 |
73 |
9 |
80 |
20 |
| 64 |
Myclobutanil |
98 |
2 |
81 |
14 |
| 65 |
Naled |
117 |
9 |
104 |
2 |
| 66 |
Novaluron |
101 |
2 |
74 |
11 |
| 67 |
Oxamyl |
102 |
10 |
94 |
3 |
| 68 |
Paclobutrazol |
97 |
4 |
89 |
6 |
| 69 |
Permethrin |
98 |
15 |
99 |
4 |
| 70 |
Phenothrin* |
84 |
11 |
86 |
3 |
| 71 |
Phosmet |
92 |
5 |
90 |
2 |
| 72 |
Piperonyl butoxide |
87 |
8 |
82 |
18 |
| 73 |
Pirimicarb |
93 |
3 |
97 |
6 |
| 74 |
Prallethrin |
82 |
20 |
93 |
11 |
| 75 |
Propiconazole* |
84 |
8 |
88 |
7 |
| 76 |
Propoxur |
93 |
2 |
91 |
2 |
| 77 |
Pyraclostrobin |
113 |
8 |
85 |
5 |
| 78 |
Pyrethrins* |
106 |
12 |
90 |
6 |
| 79 |
Pyridaben |
81 |
5 |
73 |
14 |
| 80 |
Quintozene |
99 |
12 |
90 |
5 |
| 81 |
Resmethrin* |
80 |
5 |
83 |
5 |
| 82 |
Spinetoram |
110 |
10 |
108 |
10 |
| 83 |
Spinosad |
85 |
13 |
94 |
7 |
| 84 |
Spirodiclofen |
110 |
5 |
91 |
2 |
| 85 |
Spiromesifen |
110 |
8 |
106 |
3 |
| 86 |
Spirotetramat |
104 |
20 |
86 |
17 |
| 87 |
Spiroxamine |
100 |
17 |
95 |
18 |
| 88 |
Tebuconazole |
102 |
7 |
82 |
6 |
| 89 |
Tebufenozide |
92 |
13 |
91 |
4 |
| 90 |
Teflubenzuron |
109 |
18 |
80 |
4 |
| 91 |
Tetrachlorvinphos |
87 |
17 |
85 |
7 |
| 92 |
Tetramethrin* |
77 |
8 |
101 |
6 |
| 93 |
Thiacloprid |
92 |
3 |
98 |
7 |
| 94 |
Thiamethoxam |
91 |
2 |
93 |
3 |
| 95 |
Thiophanate-methyl |
120 |
7 |
95 |
2 |
| 96 |
Trifloxystrobin |
108 |
3 |
85 |
11 |
Pesticides marked with an asterisk (*) in Table 3 are those where recovery data was acquired at low levels of 0.1 µg/g and high levels of 1 ug/g.
This was because it is not possible to obtain recovery data at a low level of 0.02 µg/g cannot for these pesticides due to their LOQs being higher than 0.02 µg/g.
It was not possible to obtain recovery data for cypermethrin, cyfluthrin, kinoprene, and methoprene at the lower level of 0.1 µg/g because their LOQs are higher than 0.1 µg/g.
Sample Matrix Effect (Ion Suppression)
Sample matrix effects remain the main challenge when conducting LC/MS/MS analyses. This is particularly the case for cannabis analysis which must accommodate the sheer diversity and complexity of cannabis samples.
A multitude of strategies have been applied to LC/MS/MS method development in order to overcome sample matrix effects.
These strategies have included sample dilution, sample clean-up, sample matrix-matched standard calibration, the use of stable isotope internal standards, a standard addition method, the use of high efficiency UHPLC columns for improved separation and the use of alternate ionization sources.1,2,3,4,5,6,7
This article discusses the most prevalent sample matrix effect in co-extracted matrix compounds - ion suppression.
This matrix effect was evaluated by spiking an identical amount of pesticide standard and internal standard mix into extract solutions that had been diluted to various levels with acetonitrile.
Acequinocyl exhibited significant ion suppression (~80%) in the cannabis flower extract diluted 20-fold with acetonitrile (Figure 1). Extracts with a 100-fold dilution exhibited lower ion suppression.
This was around 40% for acequinocyl in comparison with acequinocyl spiked in pure acetonitrile, potentially indicating that dilution could help minimize the effects of ion suppression (Figure 1).
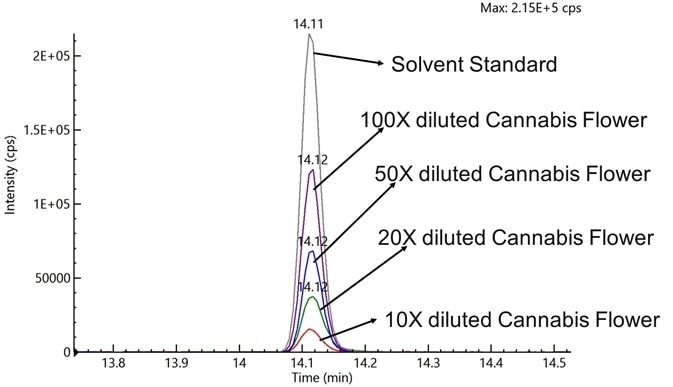
Figure 1. Chromatographic results for ion suppression at different dilution levels of cannabis flower spiked with 100 ppb of acequinocyl, and a solvent standard with 100 ppb acequinocyl. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
In the example presented here, a 20-fold dilution of the cannabis extract was utilized to minimize ion suppression of late eluting analytes from the sample matrix. This was possible without hindering the detection limits of previous eluting analytes that did not experience notable levels of ion suppression.
A total of 33 internal standards were employed to further compensate for ion suppression of analytes eluting in various regions of the chromatogram.
Internal Standards
Complex cannabis samples exhibit a severe matrix effect. To help mitigate this, 33 internal standards were used to correct any analyte loss during sample preparation and to enhance quantitative analysis and overall recovery.
Experimental results (Figure 2) indicated that the use of internal standards radically increased overall recovery in the analysis.
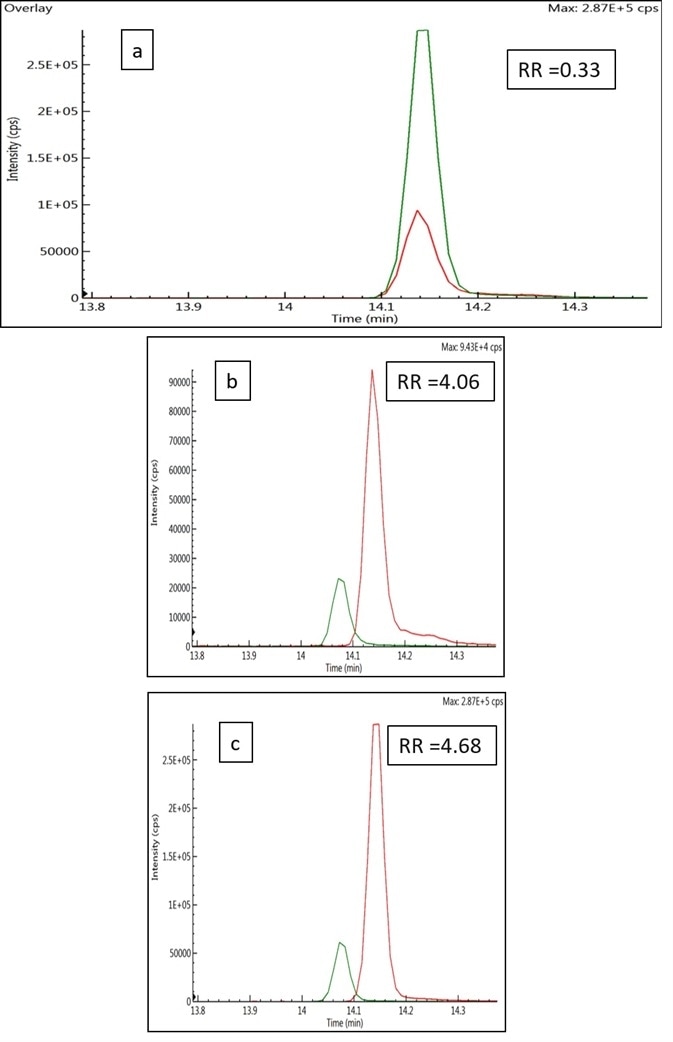
Figure 2. (a) Overlay of the responses of acequinocyl in solvent (green), and the prespiked cannabis flower matrix without internal standard (red). The response ratios of acequinocyl in cannabis extract to solvent standard was 0.33. (b) Overlay of the responses of acequinocyl (red) and the acequinocyl internal standard (green) in a pre-spiked cannabis flower matrix with response ratio (RR) of 4.06 for the analyte to the internal standard. (c) Overlay of the responses of acequinocyl (red) and acequinocyl internal standard (green) in solvent with response ratio (RR) of 4.68 for the analyte to the internal standard. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
The recovery improved from 33% to 86.5% when calculated as the extracted concentration of pre-spiked analyte versus the neat solution (unextracted) concentration. This was due to analyte loss and the correction of matrix effects during the extraction step.
Overall recoveries of 70-120% were achieved for 97% of the analytes, while recoveries of 60-140% were reported for the other 3% of analytes.
PerkinElmer offers a standard operating procedure (SOP) that details the necessary internal standards, method parameters and reagent suppliers. This is ideal for expediting validation.
LC/MS/MS Method With Optimum MRM Transitions – Ideal for Challenging Analytes
Cannabis is a challenging matrix to test, and these challenges are compounded by low concentration levels of pesticides. Optimum confidence in analyses was ensured through the use of MRM transitions for pesticides where minimal matrix interference in the cannabis matrix was determined for low level detection.
For example, it is possible to easily ionize propiconazole as a protonated molecular ion in a standard, but the MRM transitions – which are based on monoisotopic mass ion in the cannabis matrix - indicated a poor LOQ of 0.2 µg/g.
MRM transitions based on other masses were determined to reduce matrix interference, successfully achieving a LOQ of 0.04 µg/g for propiconazole.
Figure 3 displays the signal overlay of the blank cannabis matrix and propiconazole spiked at a level of 0.1 µg/g. It also shows MRM transitions with and without matrix interference, revealing that optimum propiconazole MRM transitions helped minimize matrix interference, contributing to lower detection limits.
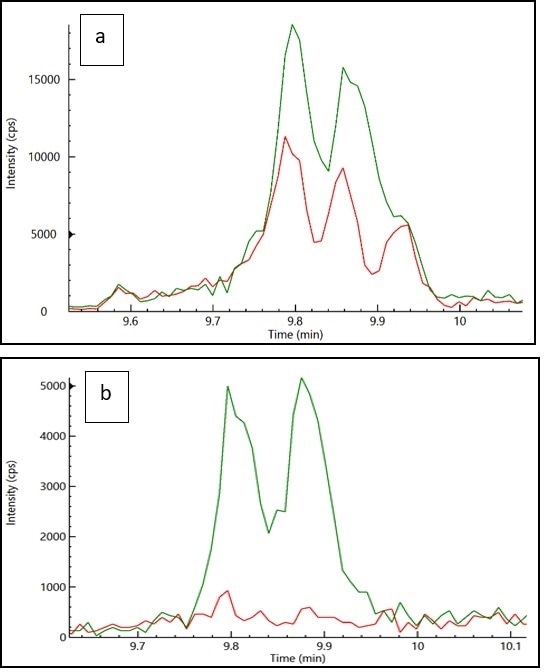
Figure 3. (a) Overlay of the response of the cannabis matrix (red) and propiconazole (green) spiked at a level of 0.1 μg/g in cannabis matrix using (a) MRM transition with matrix interference and (b) MRM transition without matrix interference. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Analysis of Pesticides via APCI
Hydrophobic and halogenated pesticides, such as chlorfenapyr and pentachloronitrobenzene, are typically analyzed using GC/MS/MS as these pesticides do not ionize effectively when using LC/MS/MS with an ESI source.
Pentachloronitrobenzene (PCNB) contains no hydrogen atoms (for loss of protons). It also contains no functional groups with high proton affinity or groups which can form ammonia or sodium adducts. These factors mean that it cannot be ionized with the ESI source.
An APCI ion source is generally more appropriate for the ionization of very hydrophobic or non-polar analytes. APCI was there employed in the determination of the detection limits of pentachloronitrobenzene, methyl parathion endosulfan-alpha, endosulfan-beta, iprodione, chlorfenapyr, fenvalerate, endosulfan sulfate and etridiazol.
Excellent signal to noise (S/N >= 100) was achieved for pentachloronitrobenzene (PCNB) spiked at a level of 0.1 µg/g using an LC/MS/MS system equipped with an APCI source and a rapid 6 minute short LC gradient (Figure 4).
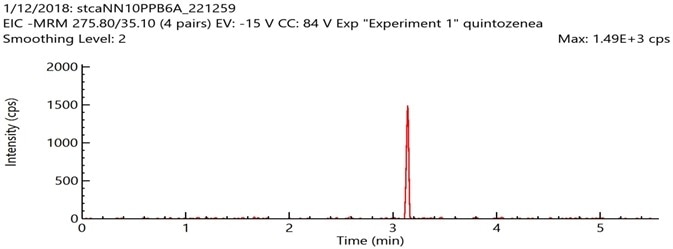
Figure 4. Sample chromatogram of pentachloronitrobenzene (PCNB) spiked at a level of 0.1 μg/g in a cannabis flower matrix using LC/MS/MS system with APCI source. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Stability Studies
Method stability was investigated by carefully monitoring 500 sample injections over the course of 1 week (Figure 5). RSDs for all analytes in APCI mode were found to be less than 20% and less than 25% in ESI mode.
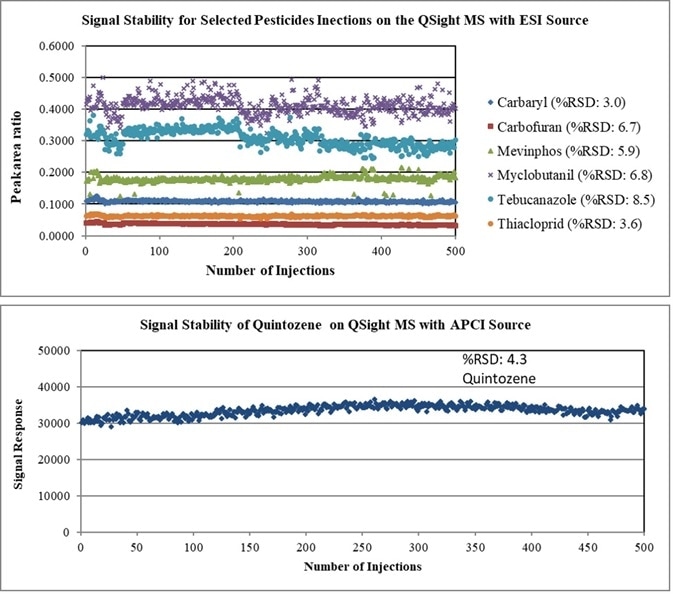
Figure 5. Long term stability data over 1 week of 500 sample injections using LC/MS/MS with ESI and APCI ion source. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
These results clearly showed that the use of the QSight LC/MS/MS system’s heated self-cleaning dual ESI/APCI ion source with laminar flow would reduce the frequent maintenance typically required when working with this dirty and challenging cannabis flower matrix.
Conclusions
This studies outlined a unique, rapid and reliable quantitative LC/MS/MS method that is ideal for the analysis of pesticide residues in cannabis samples.
The recovery of all pesticides was found to be in the acceptable range of 70-120%, with RSDs less than 20%, making this solvent extraction method an ideal choice for laboratories looking to adhere to Canadian regulations.
This method also facilitated the accurate identification and quantification of all 96 pesticides at low levels (0.0025 to 0.5 µg/g).
Its ability to screen and quantitate all 96 pesticides - including hydrophobic and chlorinated compounds typically analyzed utilizing GC/MS/MS – affords this method a clear advantage over others in the single-instrument screening and quantitating pesticides in cannabis.
References
- http://american-safe-access.s3.amazonaws.com/documents/AHP_Cannabis_Monograph_Preview.pdf .
- https://www.aocs.org/.
- K. K. Stenerson and G. Oden, Cann. Sci. & Tech., 1(1), 48-53 (2018).
- J. Kowlaski, J. H. Dahl, A. Rigdon, J. Cochran, D. Laine and G. Fagras, LCGC, 35(5) 8-22 (2017).
- X. Wang, D. Mackowsky, J. Searfoss and M. Telepchak, LCGC, 34(10), 20-27 (2016).
- L. Alder, K. Greulich, G. Kempe and B. Vieth, Mass. Spec. Rev., 25, 838– 865 (2006).
- United States Department of Agriculture Food Safety and Inspection Service, Office of Public Health Science,” Screening for Pesticides by LC/MS/MS and GC/MS/MS,” 2018, available from https://s27415.pcdn.co/wp-content/uploads/2020/01/64ER20-7/Agricultural_Agents/3-USDA-FSIS-CLG-PST5-Screening-for-Pesticides-by-LCMSMS-and-GCMSMS.pdf
Acknowledgments
Produced from materials originally authored by Avinash Dalmia, Erasmus Cudjoe, Jacob Jalali, Jeff Wu, Saba Hariri, Margaret Guthrie, Toby Astill, Charlie Schmidt, Mark Greenbaum, and Feng Qin from PerkinElmer; Ben Armstrong from Juniper Analytics; and Charlie Johnson and Kevin Smith from Napro.
About PerkinElmer Cannabis & Hemp Testing Solutions
 With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
They help drive analytical best practices and operating procedures and commit to ensuring your laboratory has maximum uptime. Learn about their various instruments, testing methods, and applications for cannabis analyses. Let them work with you to build an efficient workflow, so you can focus on growing your business.
Sponsored Content Policy: AZO Life Science publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZO Life Science, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.