Overview
The remarkable reproducibility of EpiOcular offers researchers a quantifiable evaluation of irritation. Its relation to in vivo sensitivity makes it a perfect in vitro alternative to in vivo testing for toxicological assessment of raw materials and final formulations.
EpiOcular’s powerful correlation to historical in vivo data sets and unusual reproducibility make it a perfect model for the ocular safety testing of raw ingredients and eventual formulations.
The EpiOcular Eye Irritation Test (OECD TG 492) is known to be an in vitro alternative to animal testing and is a validated OECD test guideline (TG 492). EpiOcular has been utilized for many years by the industry as a non-animal, in vitro alternative to identify Draize scores (for water-soluble test articles) and to evaluate the mildness or ultra-mildness (sub-Draize) of materials contacting the eyes.
Technology
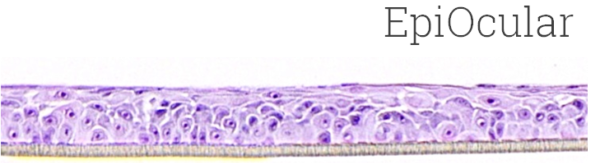
Image Credit: MatTek
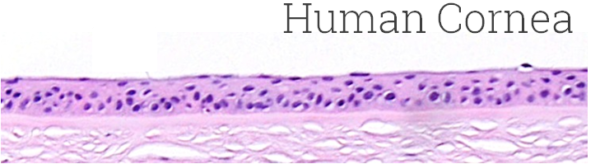
Image Credit: MatTek
Throughout the world, EpiOcular eye irritation assays have been integrated into toxicology labs. With direct protocols and easy yet highly predictive endpoints, EpiOcular assays are regularly used across the personal care, cosmetic, pharmaceutical, and chemical industries.
Developed over two decades, EpiOcular has been utilized for testing thousands of materials. If researchers require a risk assessment assay for the irritation ability of an ocular-delivered material or the risk assessment of chemicals being transported across the world, EpiOcular is the test system of choice to fulfill the ocular testing requirements of the user.
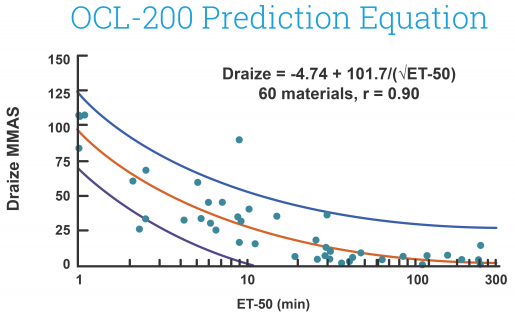
Image Credit: MatTek
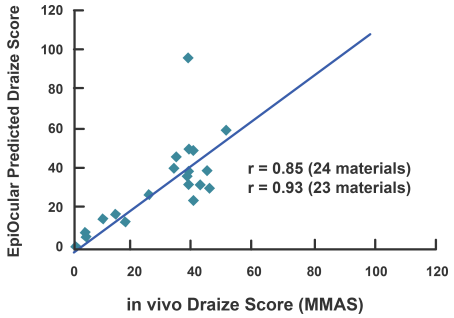
Image Credit: MatTek
Human relevance
Having years of reproducible reliability, and a recent OOECD-accepted test guideline, EpiOcular is a tested non-animal method for pharmaceutical, chemical, and personal care product testing.

Image Credit: MatTek
Applications
Ocular irritation tests
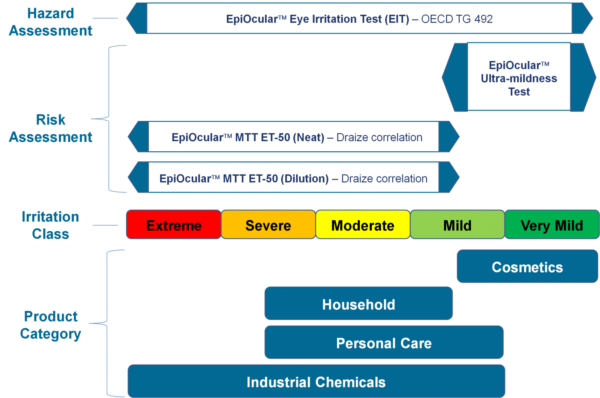
Image Credit: MatTek
EpiOcular eye irritation test (OECD TG 492)
The EpiOcular EIT is an in vitro threat assessment assay that has the potential to distinguish materials that are known to be ocular non-irritants from materials that are ocular irritants. The EIT is relevant to an extensive range of inorganic and organic chemicals and has regulatory acceptance as OECD TG 492.
EpiOcular sub Draize ultra-mildness test
The EpiOcular Ultra-mildness Test is an in vitro risk assessment assay regularly used for mild materials for which the Draize test seems to be insensitive. This highly reproducible assay enables quantifiable discrimination among mild, milder, and mildest product formulations.
EpiOcular MTT ET-50 (Neat)
This in vitro risk assessment assay depends on the effective time at which a material (applied neat) results in a 50% reduction in tissue viability (ET-50). Depending on the ET-50, the test article is classified into one of four classifications varying from non-irritating to severe or extreme which match to groupings of Rabbit Draize Eye Scores (MMAS).
EpiOcular MTT ET-50 (Dilution)
Identical to the MTT ET-50 Neat technique, this in vitro risk assessment assay is based on the efficient time at which a material (diluted to 20% in water) makes a 50% decrease in tissue viability (ET-50).
Depending on the ET-50, the test article has been classified into one of four classifications varying from non-irritating to severe or extreme, which resemble groupings of Rabbit Draize Eye Scores (MMAS). This technique is suggested for surfactant-based solutions and is relevant to water-soluble materials consisting of a specific gravity of >0.95.