At the Chalmers University of Technology, scientists have demonstrated that the Atox1 protein, which is highly concentrated in breast cancer cells, take part in a process—the process through which cancer cells metastasize.
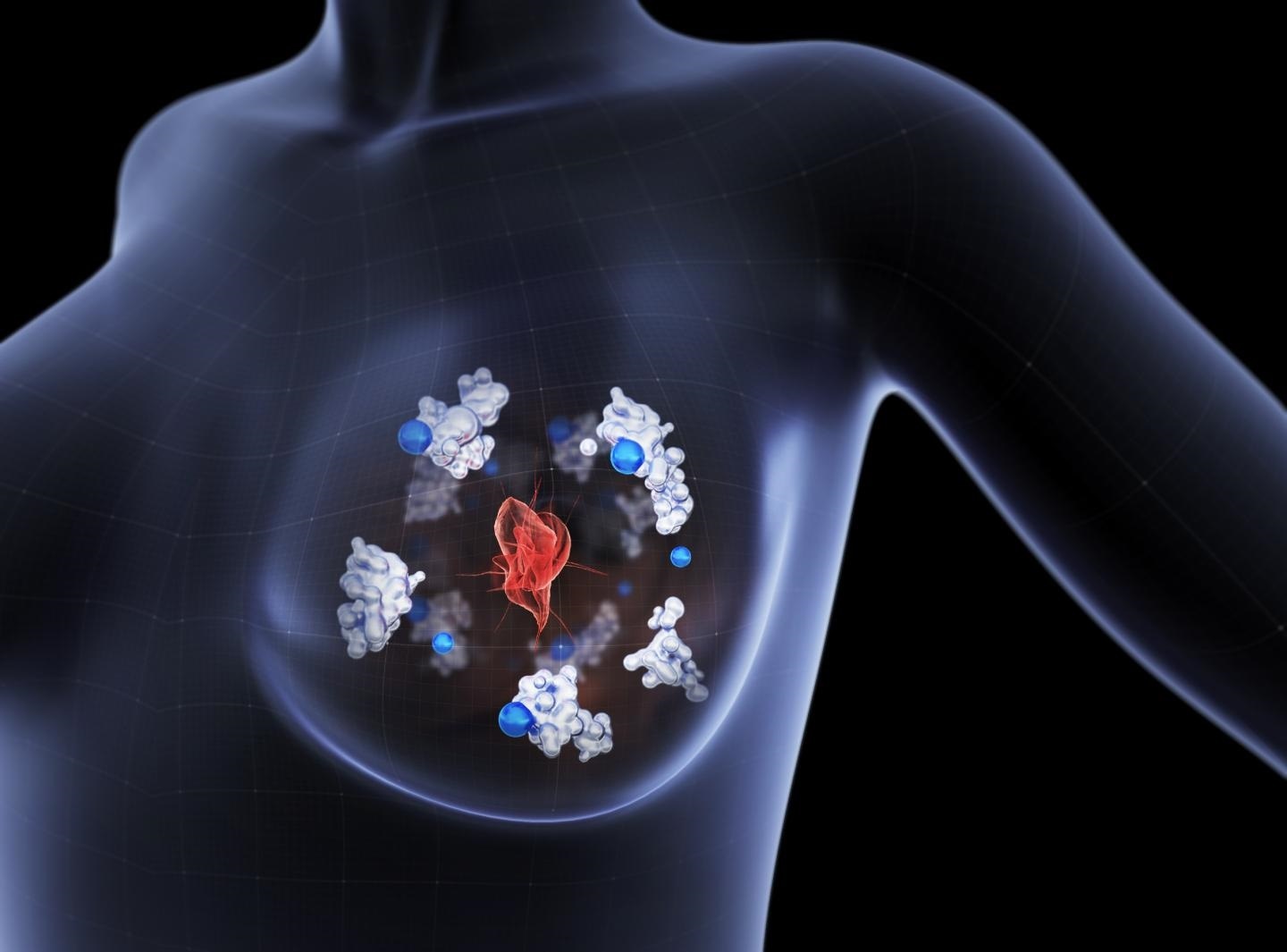
The copper-transporting protein Atox1 (illustrated in white) delivers copper (blue) to an enzyme which facilitates breast cancer cells to migrate away from the primary tumor (red) and metastasize towards other parts of the body. Image Credit: Boid.
This implies that the protein can be a potential biomarker for evaluating the aggressiveness of breast cancer, and can also serve as a promising target for novel drugs. The study was recently published in the PNAS journal.
Globally, the most common form of cancer in women is breast cancer. The survival rate crucially depends on early diagnosis and treatment.
A majority of the deaths associated with breast cancer are often caused by the migration of cancer cells, that is, exiting the primary tumor and metastasizing in other different parts of the body, such as the lungs, liver, or skeleton.
However, the molecular mechanisms responsible for the migration of cancer cells to other parts of the body are not yet been fully understood.
Breast cancer coincides with higher levels of copper in the tumors
Earlier studies have demonstrated that, similar to other types of cancers, breast cancer corresponds with higher concentrations of copper both in the blood and the tumor cells of the patients; however, the application of this additional copper in cancer cells is yet to be known.
Many metal ions, including copper, are crucial for several biological functions, in minute and controlled amounts. Since free copper ions are lethal, all copper in the human body is coupled to proteins. Copper absorption occurs through food and this element is subsequently transported to other parts of the body through transport proteins.
Atox1 is localized at the leading edge of migrating cancer cells
At the Chalmers University of Technology, scientists have currently detected a copper-binding protein that evidently impacts the migration of breast cancer cells.
There are clinical trials where they use copper depletion as a therapeutic strategy, but we focus on the copper-binding proteins as potential targets. Using a database, we first identified all the different copper-binding proteins in humans and then we compared the amount of these proteins in cancerous to healthy tissues. Atox1 was one of the copper-binding proteins with a high concentration in breast cancer cells.”
Pernilla Wittung-Stafshede, Professor of Chemical Biology, Department of Biology and Biological Engineering, Chalmers University of Technology
The so-called copper-transporter, Atox1, is a protein that delivers copper to other proteins present in human cells. These cells need copper for enzymatic functions.
The research team at the Chalmers University of Technology recently discovered that Atox1 is localized at the leading edge of moving cancer cells.
This implies that the protein is likely to be involved in the movement of cells. Such an observation became the starting point for the study.
The researchers tracked the movement of cancer cells
With the help of sophisticated live-cell video microscopy, the team successfully observed and monitored the pattern of movement of an unlimited number of individual cancer cells, both with and without the presence of the Atox1 protein.
Nobody has studied how a copper-binding protein affects migration of breast cancer cells before. This is a high-resolution method and the experimental work has been time consuming, but we got a result that is very pure and informative. We were able to demonstrate that the cells moved at higher speeds and over longer distances when Atox1 was present, compared to the same cells having less of the protein.”
Stéphanie Blockhuys, Study First Author and Postdoctoral Researcher, Department of Chemical Biology, Chalmers University of Technology
Atox1 drives cell movement
Additional experiments show that the Atox1 protein drives the movement of cells by activating a chain of reactions comprising the enzyme lysyl oxidase (LOX) and another copper transport protein called ATP7A.
The Atox1 protein transports copper to ATP7A, which consequently transports the metal to LOX in a synchronized reaction.
LOX can function only with the help of copper, and it is already known that this enzyme plays a role in extracellular processes that facilitate the movement of breast cancer cells.
When Atox1 in the cancer cells was reduced, we found extracellular LOX activity to be decreased. Thus, it appears that without Atox1, LOX does not receive the copper required for its cell migration potential.”
Stéphanie Blockhuys, Study First Author and Postdoctoral Researcher, Department of Chemical Biology, Chalmers University of Technology
High Atox1 levels drastically influence survival
The scientists simultaneously examined a database of Atox1 transcript levels reported in as many as 1904 different breast cancer patients, together with survival times. The team discovered that patients whose tumors have increased levels of Atox1 have significantly lower survival times.
Thus, the researchers concluded that the mechanism which they detected in their cell culture experiments appears to be involved in the progression of breast cancer in patients. This shows that the Atox1 protein could be a potential biomarker for evaluating the extent of the aggressive nature of breast cancer.
Such data could be utilized, for instance, to find out whether treatment to remove copper from the body could be suitable. In addition, the Atox1 protein could become a target drug for inhibiting metastasis, and thus the death of cancer patients.
“What we have found could be important for all types of cancer. How cancer cells move is a fundamental process of cancer metastasis that we still don’t understand well enough,” concluded Pernilla Wittung-Stafshede.
The scientists will currently transfer the experiments from cells to tiny animal models and analyze whether or not other copper-binding proteins are also involved.
Source:
Journal reference:
Blockhuys, S., et al. (2020) Single-cell tracking demonstrates copper chaperone Atox1 to be required for breast cancer cell migration. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.1910722117.