.jpg) By Dr. Chinta SidharthanReviewed by Lexie Corner
By Dr. Chinta SidharthanReviewed by Lexie CornerInfectious disease control, vaccine durability, and immune-based therapies all rely on one key principle: immune memory.
Understanding how the immune system remembers past encounters with pathogens—and how it responds to reinfections—is essential for developing better public health strategies.
 Image Credit: Motortion Films/Shutterstock.com
Image Credit: Motortion Films/Shutterstock.com
What Triggers Long-Term Immune Memory
Immune memory is a defining feature of the adaptive immune system. It forms the basis of vaccination and explains why people who recover from an infection are often better protected if exposed again to the same pathogen.
Unlike innate immune responses, which act quickly and uniformly, adaptive responses are highly specific. They also provide long-lasting protection. This protection is driven by memory B cells and T cells. These cells retain information about past infections and enable a faster, stronger response when the same pathogen returns.1,2
Studying the cellular and molecular basis of immune memory helps us understand how vaccines work. It also guides the development of better strategies to protect against persistent and emerging infections.
Download your PDF copy now!
Cells Involved in Immune Memory
Memory B Cells
Memory B cells are key components of humoral immunity. They develop from germinal center reactions after exposure to an antigen. These cells undergo somatic hypermutation and isotype switching, which produces high-affinity, antigen-specific clones. When exposed again to the same antigen, memory B cells quickly differentiate into antibody-secreting plasma cells. This allows for rapid neutralization of the pathogen.2
Some subsets, such as immunoglobulin (Ig)M⁺ memory B cells and B1 cells, have memory-like functions without needing help from T cells. This expands the concept of B cell memory beyond traditional humoral immunity, particularly during early responses to infection.2,3
Memory T Cells
Memory T cells include both CD4⁺ (helper) and CD8⁺ (cytotoxic) subsets. These cells provide long-term cellular immunity. After initial antigen exposure, naive T cells expand into effector cells. Some of these persist as memory T cells.
Memory T cells fall into three main categories: central memory, effector memory, and tissue-resident memory T cells.4 These subsets differ in localization, migratory patterns, and functionality.
Central memory T cells reside in lymphoid tissues and exhibit high proliferative capacity, whereas effector memory cells patrol peripheral tissues and mount rapid effector functions. Tissue-resident memory T cells remain in previously infected sites and are crucial for frontline defense at mucosal barriers.4,5
The diagram below illustrates the main types of T cells, including those involved in immune memory.
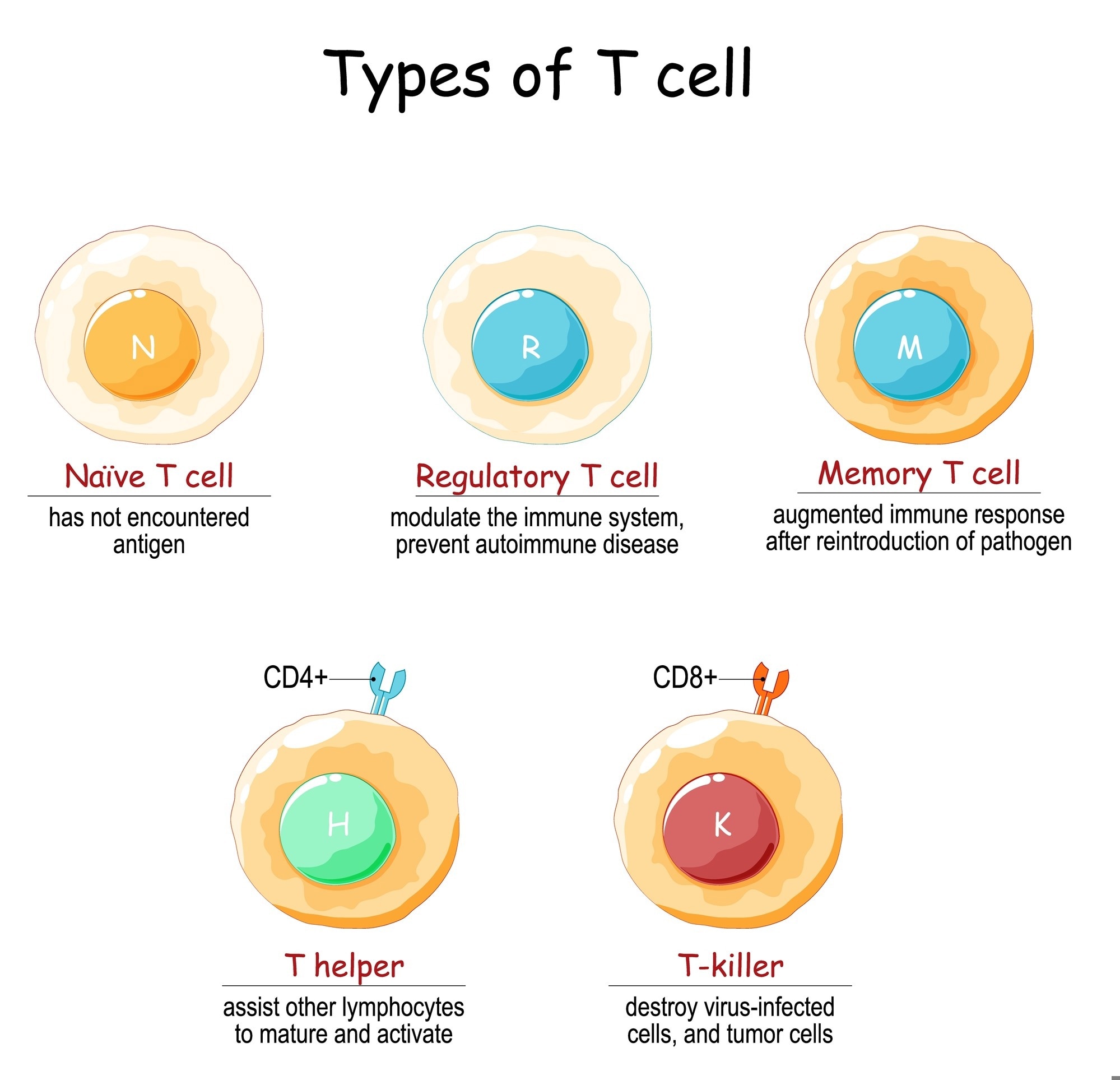
Naïve T cells have not yet encountered antigen. Regulatory T cells modulate immune responses and help prevent autoimmunity. Memory T cells mount a faster and stronger response upon re-exposure to a pathogen. Helper T cells (CD4⁺) support the activation and maturation of other immune cells, while cytotoxic T cells (CD8⁺) target virus-infected and tumor cells. Image Credit: Designua/Shutterstock.com
Natural Killer Cells and Myeloid Lineages
Despite earlier assumptions, natural killer (NK) cells and some other innate immune cells can also show memory-like behavior. NK cells exposed to viral antigens or inflammatory signals can undergo clonal expansion and persist long-term. When re-stimulated, they respond more strongly. This has been well documented in models of cytomegalovirus infection, where antigen-specific NK cell memory provides robust protection.6
Monocytes and macrophages can also display a form of immune memory called "trained immunity." This involves epigenetic and metabolic changes that enhance responses to later stimuli. These responses do not rely on specific antigens and can occur even with unrelated pathogens.7
The Molecular Basis of Immune Memory
The durability and rapid responsiveness of memory lymphocytes are supported by specific molecular changes. In T and B cells, memory formation involves epigenetic modifications, including deoxyribonucleic acid (DNA) demethylation and histone acetylation. These changes keep important effector genes ready for rapid activation.1
For instance, memory T cells show enhanced accessibility to genes coding for cytokines such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), facilitating swift transcriptional responses upon re-encounter with the antigen.1
Metabolic changes also support memory cell function. Memory T cells rely more on oxidative phosphorylation and fatty acid oxidation. These pathways contribute to their longevity and functional fitness.8
In innate immune cells, a similar process occurs. Trained immunity, found in cells like NK cells and monocytes, involves epigenetic changes triggered by exposure to microbial signals such as β-glucans or the Bacillus Calmette-Guérin (BCG) vaccine. This results in stronger cytokine responses when the cells are stimulated again.8
Why Vaccines Work: The Role of Immune Memory
Vaccines are designed to imitate natural infections in a controlled way. Their goal is to create long-lasting immune memory without causing illness. They do this by activating dendritic cells, which present antigens to T and B cells. Adjuvants and improved delivery systems help strengthen this process.
These tools shape the innate immune response and support the formation of germinal centers, where memory cells are generated.4
Still, immune memory does not always provide sterilizing immunity. In some cases, memory responses cannot prevent reinfection, especially at mucosal surfaces, where pathogens may replicate before memory cells can respond. This gap shows that having immune memory is not the same as being fully protected. It also highlights the importance of developing mucosal vaccines or strategies that produce tissue-resident memory cells.4
Some viruses, including HIV, influenza, and hepatitis C, can escape immune memory. They do this by mutating rapidly or interfering with how antigens are presented.5 These features make vaccine development more difficult. Understanding how memory cells respond to altered or new antigens is key to creating broadly protective (or “universal”) vaccines.
To learn more about how vaccines are designed to trigger immune memory, visit: What are the Different Types of Vaccines?
What We’re Still Learning About Immune Memory
New tools like single-cell sequencing, epigenomic profiling, and metabolic analysis are helping researchers better understand immune memory, but key questions remain. How do memory B and T cells maintain long-term survival? How do different memory subsets contribute to protection or disease? How can memory responses be improved for chronic or evasive infections?1,8
Researchers are also studying non-traditional forms of memory, such as trained immunity in monocytes and innate lymphoid cells, in vaccine responses and host defense.1,8
As our understanding grows, vaccination strategies may become more personalized. Vaccine formulations could be tailored to factors like age, genetics, or prior exposure to improve memory formation.
Another promising area is the use of targeted delivery systems or mucosal immunization to activate tissue-resident memory T cells. This approach may enhance protection at the sites where many infections begin.
As researchers continue to explore the many layers of immune memory, our ability to design more effective, personalized immunotherapies will grow.
For a deeper look at where immunology is headed, watch 2024 the WMIF panel on the Current and Future States of Immunology:
2024 WMIF | Current and Future States of Immunology
References and Further Reading
- Natoli, G., Ostuni, R. (2019). Adaptation and memory in immune responses. Nature Immunology, 20(7), 783–792.
DOI:10.1038/s41590-019-0399-9
- Kurosaki, T., Kometani, K., Ise, W. (2015). Memory B cells. Nature Reviews. Immunology, 15(3), 149–159. DOI:10.1038/nri3802
- Lau, C. M., Sun, J. C. (2018). The widening spectrum of immunological memory. Current Opinion in Immunology, 54, 42–49. DOI:10.1016/j.coi.2018.05.013
- Campos, M., Godson, D. L. (2003). The effectiveness and limitations of immune memory: understanding protective immune responses. International journal for parasitology, 33(5-6), 655–661. DOI:10.1016/s0020-7519(03)00066-3
- Mueller, S. N., Rouse, B. T. (2008). Immune responses to viruses. Clinical Immunology, 421–431. DOI:10.1016/B978-0-323-04404-2.10027-2
- Cerwenka, A., Lanier, L. L. (2016). Natural killer cell memory in infection, inflammation and cancer. Nature Reviews. Immunology, 16(2), 112–123. DOI:10.1038/nri.2015.9
- Domínguez-Andrés, J., Joosten, L. A., Netea, M. G. (2019). Induction of innate immune memory: the role of cellular metabolism. Current Opinion in Immunology, 56, 10–16. DOI:10.1016/j.coi.2018.09.001
- Gardiner, C. M., Mills, K. H. (2016). The cells that mediate innate immune memory and their functional significance in inflammatory and infectious diseases. Seminars in Immunology, 28(4), 343–350. DOI:10.1016/j.smim.2016.03.001
Last Updated: Jun 12, 2025