The antibiotic was created nearly three decades ago, but its precise mechanism of action was not clear until now.
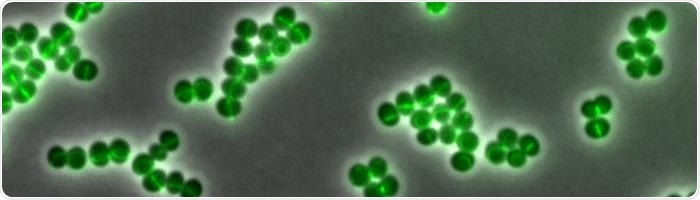
Daptomycin binds to certain areas of the cell envelope of the bacterium Staphylococcus aureus, which are particularly vulnerable. The fluorescence-labeled Daptomycin glows green. These are the areas where the cells are currently dividing. Image credit: © Fabian Grein.
With regards to the bacterial infections, antibiotics are considered the ideal medical weapons; however, they are turning increasingly blunt. The resistances for antibiotics have enormously increased, due to which several antibiotics are no longer effective against the harmful pathogens.
A few of these active substances are targeted specifically for severe infections with resistant bacteria. For instance, daptomycin, which was launched in the United States in 2003 and Germany in 2006, is helpful for the treatment of infections with methicillin-resistant Staphylococcus aureus (MRSA) and resistant enterococci.
Although daptomycin was discovered around 30 years ago, the exact mode of action remained elusive until now.”
Dr Tanja Schneider, Professor, Institute of Pharmaceutical Microbiology, University of Bonn
Dr. Schneider is also affiliated with the German Center for Infection Research (DZIF). Several hypotheses were made on how the antibiotic attacks and destroys bacteria. According to one theory, daptomycin penetrates the envelope of bacteria and causes potassium efflux, which results in the death of the bacterium.
According to Anna Müller, one of the lead authors from Prof. Schneider’s research group, “Nobody understood how daptomycin actually works.”
Interdisciplinary research team
The multidisciplinary team from the fields of medicine, physical chemistry, and pharmacology employed a broad array of scientific techniques to find out the antibiotic’s mechanism of action. Initially, the scientists labeled daptomycin with a fluorescent dye that glows green.
The labeling allowed them to track precisely where the antibiotic docks to the staphylococcal cells when observed under a high-resolution microscope.
Daptomycin binds to the bacteria in regions where the new cell wall is just being synthesized.”
Dr Fabian Grein, Study Co-Lead Author, University of Bonn
Dr. Grein is Prof. Schneider’s colleague. Similar to a construction kit, the wall of the bacterial cell is formed from several building blocks.
In-depth analyses on staphylococci and synthetically created bacterial walls revealed that two of these building blocks are specifically very significant for the effect of daptomycin—the membrane lipid phosphatidylglycerol (PG) and the central cell wall building block “lipid II.”
According to Schneider, “The combination of lipid II and PG together is the Achilles' heel of the bacteria.” Daptomycin plays a vital role precisely in this phase. The antibiotic captures these vital building blocks and inhibits the further construction of the cell wall.
Consequently, the bacterial cell wall turns unstable, thus leading to the outflow of different ions, including potassium.
“The outflow of ions is not the actual killing mechanism of daptomycin, as originally thought, but a consequence of bacterial cell death,” concluded Schneider.
We were able to show how daptomycin really works and to which molecular target structures it docks.”
Dr Ulrich Kubitscheck, Professor, Department of Biophysical Chemistry, University of Bonn
It is one of the crucial prerequisites for further enhancement of daptomycin. Scientists have been focusing to develop combination therapies with various active compounds because these compounds cannot be developed to the preferred extent to combat antibiotic resistance.
Prof. Schneider explained, “The strategy is to target already resistant bacteria with differently acting weapons.” But this strategy would hold well only if the mechanism of action and targets of the antibiotics are known.
The research was conducted in the Transregional Collaborative Research Center TRR261 “Antibiotic Cell Map—Cellular Mechanisms of Antibiotic Action and Production” located at the Universities of Bonn and Tübingen and financially supported by the German Research Foundation (DFG).
Schneider said, “It was only through this transdisciplinary cooperation that we were able to take the decisive step forward and solve a puzzle that science has been mulling over for 30 year.”
Source:
Journal reference:
Grein, F., et al. (2020) Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nature Communications. doi.org/10.1038/s41467-020-15257-1.