The word “hangry” describes individuals who become angry due to hunger. According to a recent study by Adam Rosenthal, PhD, an assistant professor in the department of microbiology and immunology, some bacteria cells experience hangry and release toxic substances into the body when they are denied certain nutrients.
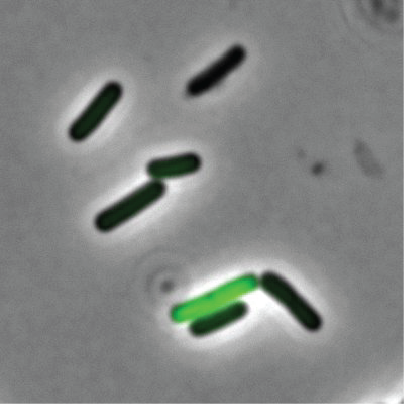 A fluorescent microscope image shows that in a population of genetically identical. The cell in green is expressing a green-fluorescent protein that is expressed by cells that are able to take up DNA from the environment. Image Credit: Rosenthal et. al
A fluorescent microscope image shows that in a population of genetically identical. The cell in green is expressing a green-fluorescent protein that is expressed by cells that are able to take up DNA from the environment. Image Credit: Rosenthal et. al
Using a recently developed technology, Rosenthal and his colleagues from Harvard, Princeton, and Danisco Animal Nutrition observed that genetically identical cells within a bacterial community have distinct purposes, with some members behaving more docile and others producing the very toxins that make people feel ill.
Bacteria behave much more different than we traditionally thought. Even when we study a community of bacteria that are all genetically identical, they don’t all act the same way. We wanted to find out why.”
Adam Rosenthal, PhD, Assistant Professor, Department of Microbiology and Immunology, UNC School of Medicine
The results, which were published in Nature Microbiology, are crucial for understanding how and why bacterial communities delegate tasks to specific cells. They could additionally suggest new approaches to combating antibiotic tolerance in the future.
Rosenthal decided to look more closely at the differences between cells that behave as “well-behaved citizens” and those that are “bad actors” and are responsible for releasing toxins into the environment.
He chose Clostridium perfringens, a rod-shaped bacterium that lives in dirt, insects, and the intestinal tracts of humans, other animals, and other vertebrates, as the microbe of his study.
They were able to partition or separate single bacterial cells into droplets using a gadget known as a microfluidic droplet generator, which allowed them to decipher each and every cell.
The cells of C. perfringens that were not producing toxins were discovered to be well-fed with nutrients. However, it appears that those essential nutrients are not present in the cells of C. perfringens that produce toxins.
Rosenthal postulated, “If we give more of these nutrients maybe we can get the toxin-producing cells to behave a little bit better.”
The researchers then subjected the bad actor cells to acetate. Their theory proved correct. Not only did toxin levels decrease throughout the community, but so did the number of bad actors. But, in the aftermath of such astounding findings, even more concerns have arisen.
Rosenthal wonders whether there are specific environmental variables that could be ‘turning on’ toxin production in other types of infections or if this new discovery is only true for C. perfringens now that it is known that nutrients play a major role in toxicity.
The most significant claim made by Rosenthal is that feeding bacteria with nutrients could result in a brand-new treatment option for both humans and animals.
For instance, the strong adversary in the hen house is the model organism Clostridium perfringens. Antibiotic use is declining in the food industry, leaving poultry helpless against the deadly, quickly contagious disease. Farmers could soon have a new tool to decrease pathogenic microbes without using antibiotics with the latest results from Rosenthal et al.
There is still more to be done for people. To adapt his latest results to address antibiotic tolerance, Rosenthal is currently collaborating with colleagues from across UNC.
When some bacteria are able to avoid the drug’s intended target despite the community not having undergone mutations that would have made all cells immune to an antibiotic, this is known as antibiotic tolerance. A less-effective treatment could result from this type of tolerance, but it is unclear what controls it.
Rosenthal will carry on studying these more intricate bacterial communities in the interim to learn more about their motivations.
Source:
Journal reference:
McNulty, R., et al. (2023). Probe-based bacterial single-cell RNA sequencing predicts toxin regulation. Nature Microbiology. doi.org/10.1038/s41564-023-01348-4