Although bacteria are typically thought of as single-celled creatures suspended in liquids or dispersed sparsely across surfaces, their true mode of growth is in sticky clusters known as biofilms in many settings.
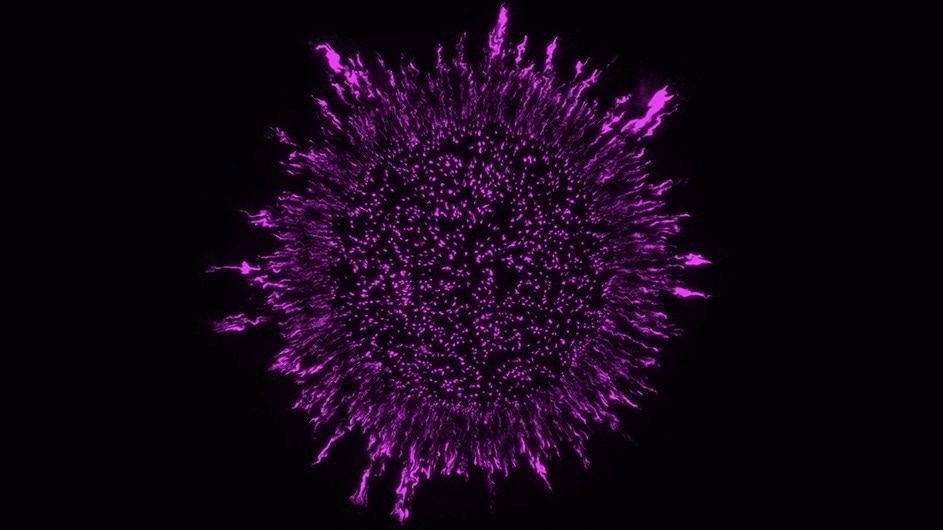
Image Credit: Columbia University
Humans can benefit from biofilm formation; for instance, it is necessary to make kombucha tea. However, because it makes it more challenging to manage bacterial development, it is more frequently problematic: Bacteria become more resistant to drugs when they form a biofilm, which serves as a barrier against external invaders.
As far as biofilm structure was concerned, until recently, experts had considered that bacteria were placed fairly randomly in biofilms. However, fresh findings from the lab of Biology Professor Lars Dietrich reveal that the bacteria that create biofilms have a highly ordered configuration inside those slimy matrices.
Their surprising discovery may open the door to creating novel medications that more effectively combat antibiotic-resistant bacteria.
There is a yin-yang trade-off for bacteria that form biofilms, since the biofilm guards against antibiotics and other threats, but also prevents food from entering and feeding the system, this research gives us an important foundation for understanding how to affect bacterial-cell arrangement and assess how to make them more susceptible to antibiotics.”
Lars Dietrich, Professor and Study Lead Author, Department of Biological Sciences, Columbia University
The latest study, led by Graduate Student Hannah Dayton and published in the journal PLOS Biology, describes the work done in Professor Dietrich's lab. The study focused on Pseudomonas aeruginosa, a significant and widespread pathogen.
The group conducted their investigation using fluorescence and scanning electron microscopy in conjunction with cell labeling. Researchers discovered that P. aeruginosa cells are stacked longitudinally and oriented perpendicular to their growth substrate—the substance the bacteria eat to stay alive and grow in biofilms. They also discovered that this arrangement is upset by mutations that alter the bacterial cell surface.
They found that the distribution of sugar influenced by the biofilm's anatomy occurred when they investigated the impacts of adding sugar from the outside to a fully developed biofilm. Certain regions inside the biofilm, called subzones, showed greater sensitivity to additional sugar or antibiotics in mutant bacteria with a disorganized cellular structure.
Lastly, they demonstrated how the site of peak metabolic activity within the structure is shifted by modifications in the anatomy of biofilms.
All of these findings suggest that the microstructure of biofilms is a feature that may be adjusted to affect the metabolism of resident bacterial subpopulations and the group's overall survival. The results have consequences for how we manage infections brought on by P. aeruginosa and other bacteria that create biofilms.
The research was conducted along with the research groups of Wei Min, a Columbia chemistry Professor; Raju Tomer, a Columbia biology professor; Jasmine Nirody, a Professor at the University of Chicago; and Anuradha Janakiraman, a Professor at the City University of New York (CUNY).
The arrangement of cells is generally an underappreciated aspect of biofilm formation, and we now know that it allows bacteria in biofilms to control their physiological states and has consequences for their survival during antibiotic treatment.”
Hannah Dayton, Department of Biological Sciences, Columbia University, New York
Dietrich concluded, “This is a promising development for the pernicious and growing problem of antibiotic-resistant bacteria.”.
Source:
Journal reference:
Dayton, H., et.al. (2024). Cellular arrangement impacts metabolic activity and antibiotic tolerance in Pseudomonas aeruginosa biofilms. PLOS Biology. doi.org/10.1371/journal.pbio.3002205