Produced in Partnership with RETSCH GmbHReviewed by Emily MageeOct 30 2025
In pharmaceutical research and production, precision, reproducibility, and meeting regulatory standards are key to success. Accurate control of particle size directly affects drug distribution, tableting, and quality assurance. With Retsch’s advanced lab solutions, your team will be well-equipped to handle these challenges confidently and maintain the highest standards of product quality.
All-in-One Solutions for Maximum Efficiency
Retsch’s new AS 200 jet pro and AS 200 jet pharma air jet sieving machines integrate sieving, weighing, and evaluation into one convenient instrument. Each model features a high-precision balance, intuitive software, and intelligent assistants such as the Weighing Assistant and the Sieve-Check barcode verification.
These devices accelerate laboratory workflows, enhance safety, and improve traceability, which are significant advantages for GMP-compliant operations.

Air Jet Sieving Machine AS 200 jet pro. Image Credit: RETSCH GmbH
Grinding: Efficient Sample Preparation and Quality Control
Retsch mills deliver decisive competitive advantages across a variety of pharmaceutical applications:
- Research and Development: Achieve targeted particle size distributions of active ingredients and excipients to optimize bioavailability and efficacy in preparations. Temperature-controlled grinding also enables the gentle processing of sensitive substances.
- Formulation development: Ensure reliable and homogenous mixing of APIs and excipients, maintain dosage form consistency and stability, and efficiently discover novel formulations through effective co-crystal screening using mixer mills or planetary ball mills.
1. Sieving: Accurate Sieving for Maximum Product Safety
Retsch air jet sieving machines enable control of the finest sample material directly within the instrument, ensuring a safe and efficient workflow. Weighing, sieving, and evaluation can be performed in a single step, eliminating sample losses and sources of errors. As such, your laboratory staff can work more productively while achieving consistently dependable results.
With its intuitive user interface, built-in PC, and calibratable scale (with a reading accuracy of 0.01 g), the AS 200 jet pro functions as a fully stand-alone device for particle size measurement. It saves valuable time, while the user-friendly software guides you step by step through both individual and standardized methods. Results can be saved directly or exported to a LIMS system for further processing.
The AS 200 jet pharma goes a step further by meeting comprehensive GMP requirements. Features like user management, password protection, audit trails, and electronic signatures ensure full security and traceability. Customizable access rights allow for flexible team workflows, and optional IQ/OQ and risk analysis packages support fast and reliable device qualification and process validation.

Air Jet Sieving Machine AS 200 jet pharma. Image Credit: RETSCH GmbH
AS 200 Jet Pharma: New Features for Increased Process Dependability
- Sieve-check: Prevent operating errors with barcode-supported sieve verification.
- Plausibility test: Automated weight control detects missing sieves or lids before analysis begins.
- Sieve series filter: Automatically adjusts recommended sieves from the Renard series when mesh sizes change.
- Weighing assistant: Supports standard-compliant sieve loading to ensure reproducible results.
- Weigh-in tolerance: Enables individual weighing with defined tolerance limits for your samples.
- Backweigh tolerance: Allows for immediate response to deviations by adjusting the setpoint.
- Sieve trend analysis: Detects wear early on, helping maintain consistent quality over time.
- Sieve analyzer trend tracking: Monitors long-term performance for reliable, repeatable outcomes.
2. Alternative Grinding
Co-Crystals – From Screening to Kilogram-Scale Production with Retsch
As a new pharmaceutical application, co-crystals provide direct access to enhanced physicochemical characteristics of active ingredients.
They are formed from an active pharmaceutical ingredient (API) and a coformer, which together form a new crystalline structure that specifically increases solubility and dissolution rate, especially relevant for poorly soluble compounds. This enhances therapeutic bioavailability and effectiveness without altering the molecular structure of the active ingredient.
Retsch mills enable efficient and reproducible design of the entire co-crystallization process, from laboratory-scale screening to kilogram-scale manufacturing:
Efficient Screening with Planetary Ball Mills
A special adapter for planetary ball mills, compatible with PM 100, PM 300, and PM 400, allows the use of disposable GC glass vials (for example, 1.5 ml) for parallel screening tests. The adapter accommodates up to 24 jars, allowing the PM 400 to test up to 64 samples simultaneously. This capability enables the investigation of multiple co-crystal combinations in a short timeframe.
Mixer Mills for Precise Analysis
Co-crystal screening is also highly effective in mixer mills. In one study, theophylline and benzamide (1:1) were processed into co-crystals using MM 400 two ml steel tubes and a suitable PTFE adapter under the following conditions:1
- Grinding time: 60 minutes
- Frequency: 30 Hz
- Eight steel tubes, each containing one six mm stainless steel grinding ball
- Four experiments without solvent and four with 20 μl ethanol
The X-ray powder diffraction patterns of the samples match the simulated reference pattern, and all signals correspond to the desired product, confirming both the reliability and reproducibility of the process. Both the MM 400 and MM 500 series can reliably implement these screening protocols using 2 ml steel tubes.

Mixer Mill MM 400. Image Credit: RETSCH GmbH
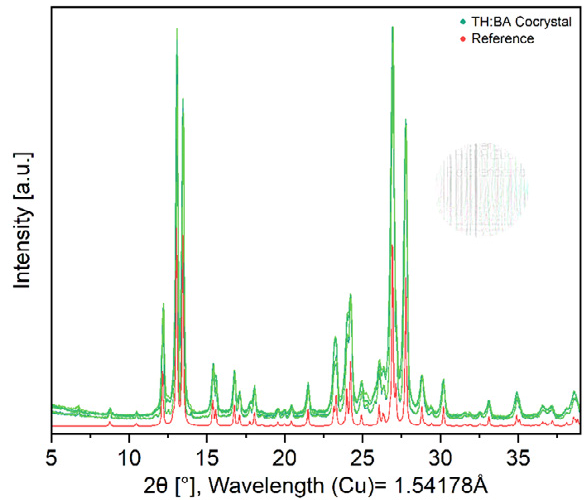
XRD pattern after co-crystal formation of theophylline and benzamide after 60 minutes of grinding in the MM 400 compared to a simulated reference. Results presented by experiments conducted by Dominik Al-Sabbagh.1 Image Credit: RETSCH GmbH
Upscaling to Kilograms
For larger-scale synthesis, the TM 300 drum mill enables efficient and sustainable production. For example, the example of rac-ibuprofen:nicotinamide co-crystal production demonstrates:
- In just 90 minutes, the TM 300 enables the production of 3.2 kg of co-crystals with a 99 % yield.
- The LAG process uses only minimal amounts of ethanol, making it an environmentally friendly choice.
- The TM 300 provides a sustainable alternative to conventional solvent-based methods.
This setup involved:
- 2.03 kg rac IBU; 1.20 kg NIC
- 10 l drum for wet grinding
- 20 kg 10 mm grinding balls stainless steel
- LAG Ethanol 0.1 ml/g
- 60 rpm for 90 minutes
- 99 % yield
One of the key advantages of the TM 300 is its extremely low metal abrasion. Measured values are well below critical thresholds and significantly lower than those of conventional eccentric vibration mills. This helps ensure maximum product purity and makes it easy to meet regulatory requirements.
Drum Mill TM 300. Image Credit: RETSCH GmbH
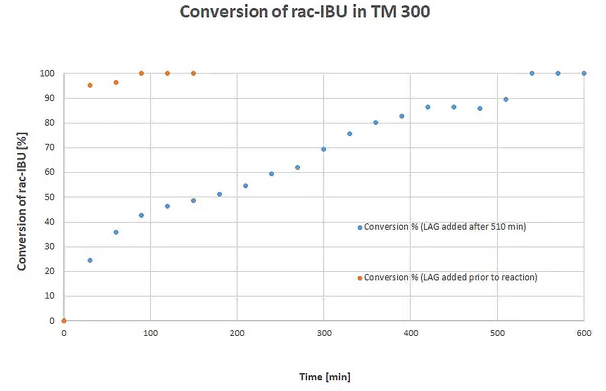
The diagram shows a conversion of rac-IBU. Blue plot: Grinding process with addition of 10 kg balls (d = 10 mm) after 270 minutes and 10 kg balls (d = 30 mm) after 360 minutes; addition of the LAG additive EtOH after 510 minutes. Orange plot: LAG-assisted process with addition of EtOH before the reaction and 20 kg of 10 mm balls. Results presented by Michael Felderhoff’s working group2. Image Credit: RETSCH GmbH
The table shows the minimum abrasion values in the TM 300 during the test. Source: RETSCH GmbH
| Sample |
Al [ppm] |
Cr [ppm] |
Co [ppm] |
Fe [ppm] |
Ni [ppm] |
| Raw material IBU |
11.3 |
39.0 |
25.7 |
71.9 |
34.9 |
| Raw material Nicotinamid |
8.9 |
33.3 |
26.7 |
40.0 |
33.3 |
| Co-crystals after 30 min |
10.8 |
35.9 |
30.8 |
51.3 |
38.5 |
| After 60 min |
11.0 |
37.0 |
31.7 |
63.4 |
39.6 |
| After 90 min |
17.2 |
43.8 |
35.9 |
64.6 |
45.3 |
Conclusion
Retsch solutions uphold the highest standards in pharmaceutical production. Alongside trusted mills for homogenization in quality control, Retsch also offers a range of innovative technologies.
The AS 200 jet pharma integrates sieving, weighing, and evaluation into a single device, boosting efficiency and ensuring reproducible results with the help of software-guided wizards and built-in check functions. Your lab teams will ultimately benefit from reliable particle separation, maximum process stability, and a precise, robust balance.
All devices are fully compliant with GMP standards, offering strong support for both quality and production control.
In the field of co-crystal technology, Retsch mills allows for rapid screening and scalable synthesis - even on a kilogram scale. This helps to ensure safe, efficient, and future-ready manufacturing processes.
References and Further Reading
- Reaktionsschema und Durchführung der Experimente: Dominik Al-Sabbagh, Chemielabortechniker, Abteilung 6.3 – Strukturanalyse, Bundesanstalt für Materialforschung und -prüfung (BAM), Berlin.
- Schöbel, J.-H., et al. (2025). Mechanochemical kilogram-scale synthesis of rac-ibuprofen:nicotinamide co-crystals using a drum mill. RSC Mechanochemistry, (online) 2(2), pp.224–229. https://doi.org/10.1039/d4mr00096j.
About RETSCH GmbH
With more than 100 years of experience in sample preparation, RETSCH is the world’s leading manufacturer of instruments for homogenizing laboratory samples for analysis as well as for analyzing the particle size of solid substances by test sieving.
The RETSCH product portfolio includes a great variety of mills and jaw crushers, capable of reducing materials down to any required fineness, sieve shakers and test sieves as well as assisting technologies to optimize the handling of samples.
RETSCH instruments stand for representative sample preparation in compliance with relevant standards and contamination-free comminution processes – they are essential tools for preparing samples for laboratory analysis and stand for reliability, precision and durability.
Since 1990 RETSCH is part of the continuously growing technology group VERDER and forms the core of the group’s Laboratory Division VERDER SCIENTIFIC. Other companies belonging to this division are Retsch Technology GmbH, ATM GmbH, Eltra GmbH and Carbolite Gero Ltd.
RETSCH company video - Solutions in Milling & Sieving
Sponsored Content Policy: AZoLifeSciences publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZoLifeSciences which is to educate and inform site visitors interested in life science news and information.