As more and more countries legalize Cannabis sp. for medicinal and recreational use, research studies continue to explore and validate its potential therapeutic effects.
Image Credit: Alpha Tauri 3D Graphics/Shutterstock.com
An example of this is the treatment of gastrointestinal diseases. The anecdotal evidence of the efficacy of cannabis in various gastrointestinal disorders has been corroborated by the discovery of endogenous cannabinoids, receptors, and synthetic and degradative enzymes in the gut.
Collectively these comprise the endocannabinoid system, whose functions include the control of tissue homeostasis, intestinal motor and sensory activity, hunger and nausea signaling, and integrity of the epithelial barrier function.
Subsequently, the use of various cannabinoid-derived pharmacological substances, such as cannabis, to treat gut-related pathologies is plausible. Underpinning these functions is a dynamic interaction with the gut microbiome. Several papers have now demonstrated a link between the gut microbiome, the endocannabinoid system, and cannabis.
What is the endocannabinoid system and how is it linked to the gut microbiome?
The human gut houses a diverse community of micro-organisms at a concentration of 1012 bacteria/ml, with its collective genetic content, or microbiome, exceeding that of the human genome over 100-fold. These bacteria contribute to human health in several ways; synthesizing essential vitamins, expanding the energy harvested from food, strengthening the gut barrier, and developing and regulating the immune system.
Similarly, the endocannabinoid system also establishes and maintains human health, exerting a systemic effect across several different systems and organs. The endocannabinoids and their receptors are distributed throughout the body and include the central and peripheral nervous systems.
There is a strong body of evidence that supports the existence of the ‘gut-endocannabinoid axis’; both gut barrier function and intestinal permeability have been shown to improve upon the addition of probiotic bacteria, mediated by the increase in select endocannabinoids concomitant with the decrease in others.
The interactions between the gut microorganisms and the endocannabinoid system, therefore, govern the integrity of the gut barrier, which directly influences health. Cannabis is one such drug that possesses the capability of bridging the endocannabinoid system and the gut microbiome.
Cannabis is a phytocannabinoid that acts on the endocannabinoid system. The most commonly researched derivatives of cannabis are Δ9 -tetrahydrocannabinol (THC) and non-psychoactive cannabidiol (CBD), which both act on the CB1 and CB2 receptors. CB1 is primarily expressed in the nervous system whilst CB2 is more restricted in its expression; found predominantly on immune cells.
With respect to the gut, CB1 is implicated in enteric nervous system function as it is found on both the submucosal neurons and myenteric neurons which provide the means of modulating intestinal motility.
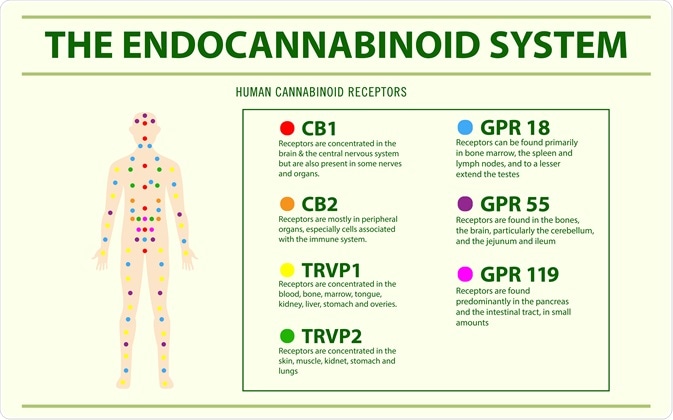
Image Credit: About time/Shutterstock.com
The effect of THC on the balance of bacterial species in the gut
In vitro assays have shown that cannabis is able to exert antimicrobial activity against a range of microorganisms. The human gut contains 4 main phyla: Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, of which Firmicutes and Bacteroidetes are proposed to propagate obesity and colorectal cancer. A
high Firmicutes: Bacteroidetes ratio is characteristic of obesity and recent evidence has demonstrated this skewed ratio can be restored in mice treated with THC. In obese mice, the gut microbiome modifies endocannabinoid signaling. The effect of this is an increase in gut permeability concomitantly with obesity-associated low-grade inflammation as well as adipogenesis. THC has been shown to prevent this effect in mice. THC demonstrably increases the abundance of the bacterial species Akkermansia muciniphila, which controls fat storage and adipose tissue metabolism to facilitate weight loss.
The bacterium also improves the endocannabinoid signaling from the gut to the brain and strengthens the gut barrier. Further, still, A. muciniphila is also associated with reduced levels of IFN-y; this reduction is known to improve glucose tolerance and thus improve control of glucose metabolism.
In humans, there is evidence to suggest that cannabis affects the microbiome. The microbiome of cannabis users has been shown to display a Prevotella/Bacteroides ratio that is 13-fold lower than that of non-users.
Building on this work, researchers have found that this diminished ratio, in combination with lower dietary intake of antioxidants and fiber, may depress mitochondrial antioxidant protection and short-chain fatty acid production which collectively result in cognitive defects. This latter point ties into existing evidence that supports the role of the Endocannabinoid System in the Brain-Gut Axis.
Demystifying the endocannabinoid system. | Ruth Ross | TEDxMississauga
Vitamin weed?
Taken together, studies suggest that an underappreciated variable in the onset of both gastrointestinal and neurodegenerative diseases is the gut microbiome. THC can change the gut microbiome, balancing it in favor of bacterial species that protect from obesity and its associated syndromes.
Such research suggests that the use of the psychoactive component of cannabis may provide a therapeutic or even preventative measure against the development of an unfavorable skew in the gut microbiome.
Sources
- Appendino, G. et al. (2008) Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod. DOI: https://doi.org/10.1021/np8002673
- Ali, E. et al. (2018) Antimicrobial activity of Cannabis sativa L.
- Cluny, N.L. et al. (2015) Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PLoS One. DOI: https://doi.org/10.1371/journal.pone.0144270
- Panee, J. et al. (2018) Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J NeuroImmune Pharmacol. DOI: https://doi.org/10.1007/s11481-017-9767-0
- Al-Ghezi, Z.Z. et al. (2017) Combination of cannabinoids, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigate experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. J Immunol. DOI: https://doi.org/10.3389/fimmu.2018.01782
- Robinson, A.P. et al. (2014) The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment, Handb Clin Neurol. DOI: https://doi.org/10.1016/B978-0-444-52001-2.00008-X
- Russo, P. (2018) Cannabis Therapeutics and the Future of Neurology. Front Integr Neurosci. DOI: https://doi.org/10.3389/fnint.2018.00051
- Elliott, D. M. (2018) Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Through Induction of Myeloid-Derived Suppressor Cells. Front Immunol. DOI: https://doi.org/10.3389/fimmu.2018.01782
Further Reading
Last Updated: Oct 28, 2020