Extracellular vesicles (EVs) are small, membrane-bound particles released by almost all cell types, and play a key role in intercellular communication by transporting biomolecules such as proteins, lipids, and nucleic acids.1
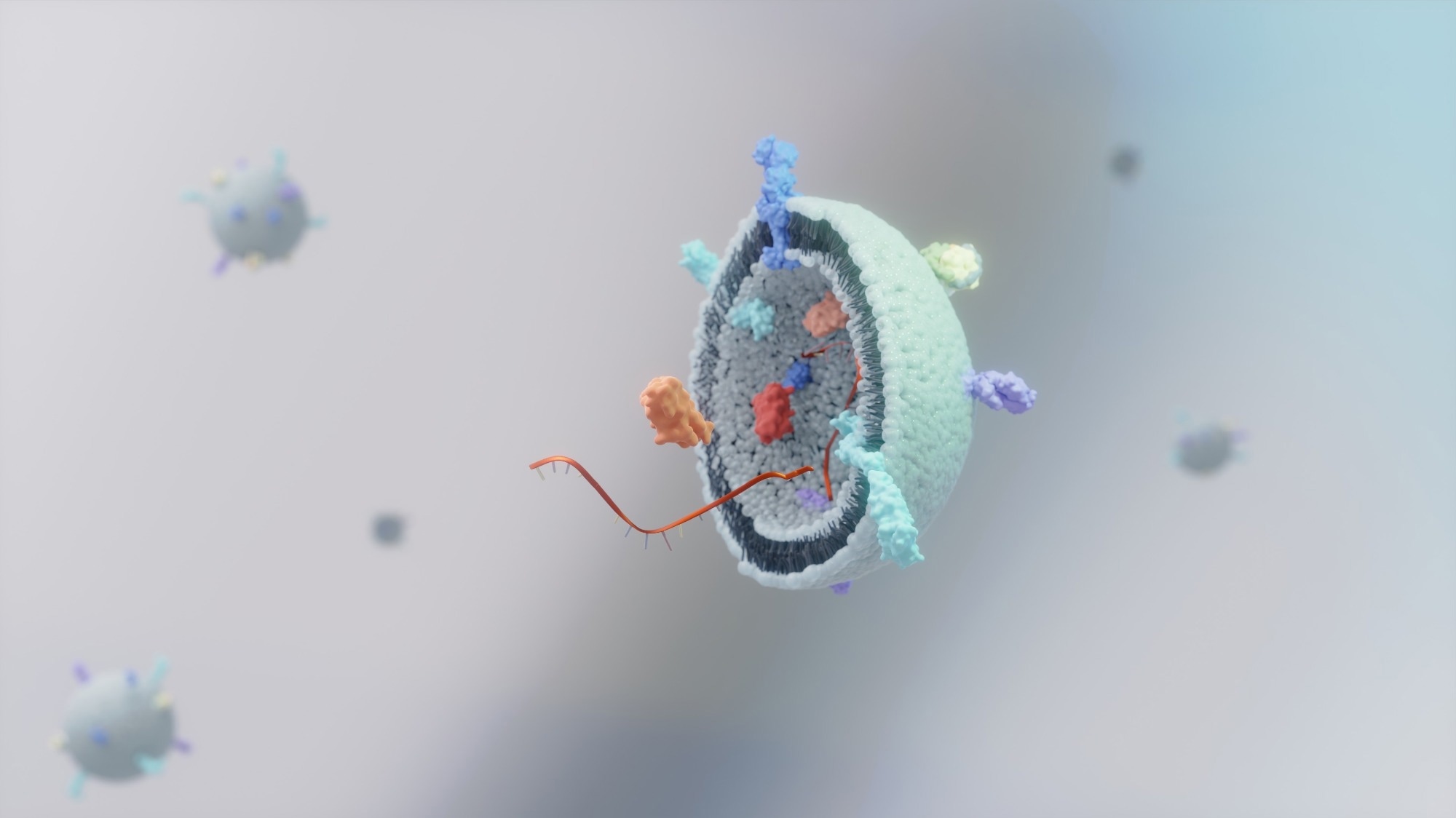 Image Credit: KwangSoo Kim/Shutterstock.com
Image Credit: KwangSoo Kim/Shutterstock.com
Introduction to Extracellular Vesicles (EVs)
EVs' natural function in cell signaling has drawn significant interest in cancer research, as tumor cells use EVs to interact with their environment, influencing processes like tumor progression and immune evasion.1
There are three subtypes of EVs: exosomes, microvesicles, and apoptotic bodies. Within the research of EVs, exosomes have been the primary focus, mainly due to their ease of derivation compared to the other subtypes.1 Thus, within the scientific literature, the terms EVs and exosomes are often used interchangeably.1,2
The potential of EVs in cancer treatment is particularly promising, as engineered EVs can deliver specific therapeutic agents or biomarkers, paving the way for personalized medicine tailored to individual patient needs and, as such, to the specific cancer type. As research advances, EVs could revolutionize cancer therapies, enhancing efficacy whilst minimizing side effects.3,4
Learn more about how analytical chemistry is used in personalized medicine
The Role of EVs in Cancer Biology
Extracellular vesicles (EVs) are crucial in cancer biology, influencing cancer development, metastasis, and immune system interactions. Acting as messengers between tumor cells and their environment, EVs assist cancer cells in communicating with nearby tissues and evading immune detection.1,5
Research has indicated that both the number and molecular content of EVs significantly change in cancerous environments, highlighting their value as biomarkers for disease detection and monitoring progression. Tumor-derived EVs carry specific proteins, lipids, RNA, and DNA that reflect the molecular profile of cancer, such as CD73 in head and neck squamous cell carcinoma or HMGB1 in hepatocellular carcinoma. 5,6
Additionally, EVs influence immune evasion by modulating the activity of immune cells, including dendritic cells and natural killer cells. Such alterations in EV quantity and cargo allow them to be promising non-invasive biomarkers for early cancer detection, prognosis, and potential therapeutic targets.5,7,8
Additionally, EVs transport cancer-related molecules, including RNA and proteins, which are vital for deciphering the tumor’s biological characteristics.9 Harnessing this capability allows for a more personalized treatment approach, allowing therapies to be customized to the specific molecular profile of each patient’s cancer.
EVs as Diagnostic Tools
Extracellular vesicles (EVs) are emerging as valuable tools for noninvasive cancer diagnostics, commonly known as “liquid biopsies.” These involve analyzing biological fluids, such as blood or urine, where EVs are present to detect cancer-related information without the need for invasive procedures.10
Cancer-derived EVs carry crucial data regarding tumor genetics and behavior, enabling early detection and continuous monitoring of treatment response. By analyzing the molecular content of these vesicles, clinicians can gain insights into tumor characteristics, assess disease progression, and evaluate how well a patient is responding to therapy. Such an approach not only enhances diagnostic accuracy but also improves the overall management of cancer patients.10
Read more advancing personalized health through bioinformatics
EVs in Drug Delivery for Personalised Treatment
In addition to their diagnostic potential, more importantly, EVs are being explored as natural, biocompatible delivery systems for cancer therapies. Given the inherent ability of EVs to transport biomolecules, they are ideal candidates for targeted drug delivery.1,11
Researchers have been engineering EVs to carry various therapeutic agents, such as chemotherapeutic drugs, RNA molecules, or immune-modulating agents, directly to cancer cells.11 The targeted method not only enhances treatment precision but also minimizes side effects related to chemotherapy, as the drugs are delivered more selectively to the tumor.12
Recent research has shown that EV-based drug delivery systems are very effective in targeting specific cancers, with great potential to improve treatment results and customize therapies. For example, a 2024 study by Bi et al. developed a new vesicle delivery system (EPM) by loading EVs from mouse breast cancer cells with the chemotherapy drug paclitaxel albumin (PA) and melanin.11
The developed nano-drug system, EPM, efficiently targeted both breast cancer cells and immune cells. During exposure to near-infrared light, the system creates signals and heat, helping immune cells (CD8+ T cells) enter tumors more effectively. 11
EPM was also found to be more toxic to cancer cells than PA alone in both lab and animal studies, showing promise for use with chemotherapy, heat therapy, and immunotherapy. Such studies highlight the growing role of EV-based approaches in personalized cancer treatment.11
EVs and Immune System Modulation
EVs can also be modified to influence the immune system, either by strengthening the body’s immune response against cancer cells or by mitigating immune suppression induced by tumors. Their inherent capacity to facilitate cell communication means they are valuable for manipulating immune responses.13
Recent progress in cancer immunotherapy has explored the use of EVs to activate immune cells or deliver tumor antigens for cancer vaccines. By promoting immune activation, these engineered EVs enhance the body’s ability to identify and combat cancer cells, ultimately leading to improved treatment outcomes and more effective immunotherapeutic approaches.13
Challenges and Opportunities in EV-Based Cancer Treatment
While promising, EV-based therapies encounter several challenges, such as difficulties scaling production, ensuring standardization, and navigating regulatory obstacles. These issues impede the progression from laboratory research to real-world clinical applications, limiting the broader implementation of EVs in cancer care and beyond for other diseases.14
Current research efforts aim to overcome these barriers, focusing on enhancing EV purification methods and improving their targeting accuracy, for example, via the modification of the EV surface or the donor cell.14,15,16
By developing more efficient techniques for producing and isolating EVs, scientists hope to create more consistent and effective treatment options. These initiatives represent significant opportunities for advancing EV-based cancer therapies, potentially resolving existing challenges, and promoting their adoption in clinical settings.
Conclusion
Extracellular vesicles (EVs) hold great potential in advancing personalized cancer treatment. Through the engineering of EVs, it facilitates targeted drug delivery, serves as biomarkers, and modulates the immune system, positioning them as innovative tools in oncology.
While challenges such as production scalability and regulatory issues persist, the potential of EV-based diagnostics and therapies to significantly enhance cancer outcomes is clear. By improving treatment precision and reducing side effects, EVs may play a crucial role in the future of cancer care, offering new hope for patients.
References
- Di Bella MA. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology (Basel). 2022;11(6). https://www.mdpi.com/2079-7737/11/6/804
- Couch Y, Buzàs EI, Di Vizio D, Gho YS, Harrison P, Hill AF, et al. A brief history of nearly EV-erything - The rise and rise of extracellular vesicles. J Extracell Vesicles. 2021;10(14):e12144. https://pubmed.ncbi.nlm.nih.gov/34919343/
- Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SEL, Vader P. Extracellular vesicles as drug delivery systems: Why and how? Advanced Drug Delivery Reviews. 2020;159:332-43. https://www.sciencedirect.com/science/article/pii/S0169409X20300247#:~:text=One%20of%20the%20most%20prominent,promising%20vehicles%20for%20drug%20delivery.
- Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduction and Targeted Therapy. 2024;9(1):27. https://www.nature.com/articles/s41392-024-01735-1
- Ahmadi M, Abbasi R, Rezaie J. Tumor immune escape: extracellular vesicles roles and therapeutics application. Cell communication and signaling. 2024 Jan 2;22(1). https://biosignaling.biomedcentral.com/articles/10.1186/s12964-023-01370-3
- Lu T, Zhang Z, Zhang J, Pan X, Zhu X, Wang X, Li Z, Ruan M, Li H, Chen W, Yan M. CD73 in small extracellular vesicles derived from HNSCC defines tumour-associated immunosuppression mediated by macrophages in the microenvironment. J Extracell Vesicles. 2022;11:e12218. https://onlinelibrary.wiley.com/doi/abs/10.1002/jev2.12218
- Wang M, Cai Y, Peng Y, Xu B, Hui W, Jiang Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020;11:896. https://www.nature.com/articles/s41419-020-03042-3
- Zhao J, Schlößer HA, Wang Z, Qin J, Li J, Popp F, Popp MC, Alakus H, Chon S-H, Hansen HP. Tumor-derived extracellular vesicles inhibit natural killer cell function in pancreatic cancer. Cancers. 2019;11:874. https://pubmed.ncbi.nlm.nih.gov/31234517/
- Patel B, Gaikwad S, Prasad S. Exploring the significance of extracellular vesicles: Key players in advancing cancer and possible theranostic tools. Cancer Pathogenesis and Therapy. 2024 Apr 25. In press, corrected proof. https://www.sciencedirect.com/science/article/pii/S2949713224000296#:~:text=Tumor%20extracellular%20vesicles%20regulate%20the,both%20immunostimulatory%20and%20immunosuppressive%20pathways.
- Irmer B, Suganja Chandrabalan, Maas L, Annalen Bleckmann, Menck K. Extracellular Vesicles in Liquid Biopsies as Biomarkers for Solid Tumors. Cancers [Internet]. 2023 Feb 18;15(4):1307–7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9953862/#:~:text=EVs%20have%20emerged%20as%20critical
- Bi Y, Chen J, Li Q, Li Y, Zhang L, Zhida L, et al. Tumor-derived extracellular vesicle drug delivery system for chemo-photothermal-immune combination cancer treatment. iScience. 2024;27(2):108833. https://www.cell.com/iscience/pdf/S2589-0042(24)00054-3.pdf
- Ma Y, Dong S, Li X, Kim BYS, Yang Z, Jiang W. Extracellular Vesicles: An Emerging Nanoplatform for Cancer Therapy. Frontiers in Oncology. 2021 Feb 8;10. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7897670/
- Greening DW, Xu R, Ale A, Hagemeyer CE, Chen W. Extracellular vesicles as next-generation immunotherapeutics. Semin Cancer Biol. 2023;90:73-100. https://www.nature.com/articles/s41565-021-00931-2
- Balachandran B, Yuana Y. Extracellular vesicles-based drug delivery system for cancer treatment. Cogent Medicine. 2019;6(1):1635806. https://biologicalproceduresonline.biomedcentral.com/articles/10.1186/s12575-023-00220-3#:~:text=Extracellular%20Vesicles%20Derived%20from%20Cells,delivery%20carriers%20for%20cancer%20treatment.
- Johnson V, V Sunil, Uday Kumar Sukumar, Kumar M. Surface-Engineered Extracellular Vesicles in Cancer Immunotherapy. Cancers. 2023 May 19;15(10):2838–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10216164/
- Danilushkina A, Emene CC, Н.А. Барлев, Gomzikova MO. Strategies for Engineering of Extracellular Vesicles. International Journal of Molecular Sciences. 2023 Aug 26;24(17):13247–7. https://www.mdpi.com/1422-0067/24/17/13247
Further Reading
Last Updated: Oct 17, 2024