G protein-coupled receptors (GPCRs) are the largest family of membrane protein receptors in humans and many other species. Based on their structure, these are also referred to as heptahelical or 7-transmembrane receptors. At present, GPCRs represent the largest number of targets for approved drugs. Some of the key factors that aided the development of GPCR-targeted drugs include their expression in the plasma membrane, druggability, and interaction with multiple types of chemical entities.1
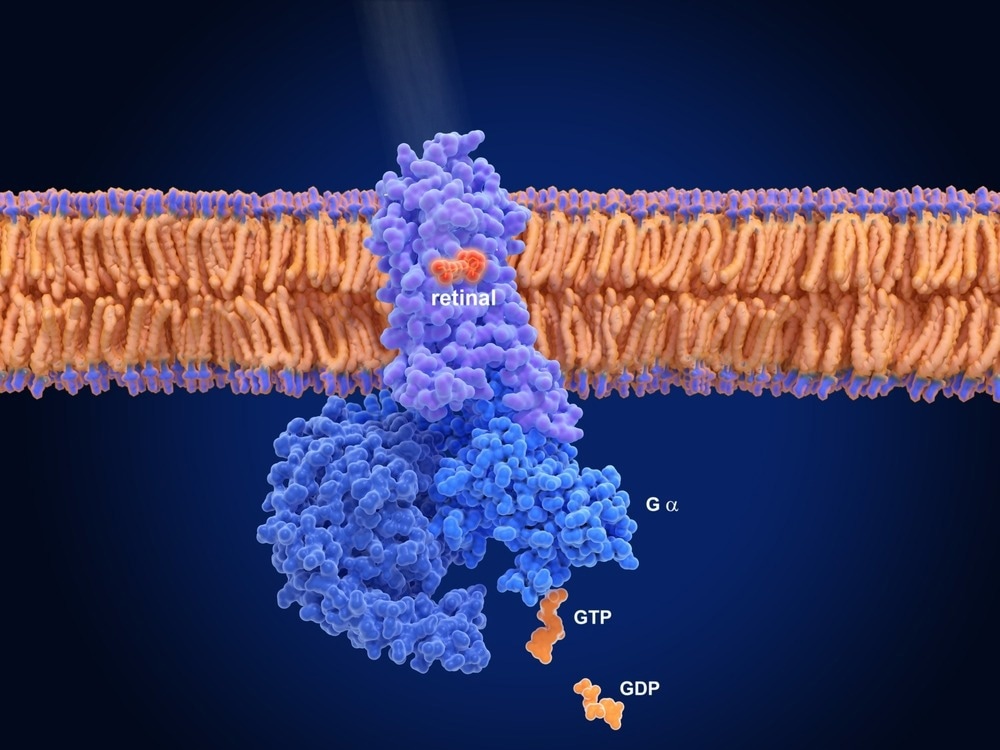
Image Credit: Juan Gaertner/Shutterstock.com
The inception of GPCR-targeted drugs was during the study of the interaction of dyes with biological structures by Paul Ehrlich (1854–1915). The finding of this study supported the idea that drugs bind to specific sites or receptors on cell surfaces. In the following years, scientists associated with GPCR research and pharmacology established the concepts of affinity and efficacy. Knowledge of agonist efficacy and the effect of signal amplification within different tissues and cells have been fundamental to the study of GPCRs.2
Structure and Functions of GPCRs
Structurally, GPCRs contain seven membrane-spanning α-helices with versatile dynamic conformations. This structure is particularly vital for extracellular stimulus recognition through the amino acids forming the extracellular loops or by the transmembrane α-helices.
Around 850 different GPCR genes have been identified in the human genome project, and half of these are considered potential drug targets. Based on amino acid sequence similarities, GPCRs are divided into six classes. Four of these classes, i.e., A, B, C, and F, are found in humans.
The majority of these receptors are activated by sensory stimuli, particularly smell. Approximately 360 GPCR genes are activated by extrasensorial ligands that include both small molecules and large proteins. Importantly, around 90% of GPCRs are expressed in the central nervous systems that are associated with major neuronal functions.3
On activation, GPCRs change their structural conformation that assists their coupling with cytosolic signal-transducing proteins, such as β-arrestin and G proteins. The conformational complexity of the GPCRs enables drugs with different chemical properties/structures to act via numerous pathways.4
GPCRs are involved with more than 80% of the signal transduction processes that occur across the plasma membrane. These receptor proteins detect a wide range of chemical signals in a highly selective manner. After detection of the signals, GPCR transduces these via ligand-receptor interactions into intracellular responses linked to vital physiological processes.
Typically, GPCR's structure can be altered through mutations, polymorphisms, and post-translational modifications, which could influence their ability to be activated by extracellular signals and the capacity to transduce important signals intracellularly.
Advancements in GPCR-targeted Ligand Screening Techniques for Drug Discovery
There are several experimental and computational methodologies to screen GPCRs-targeted drugs. Both the pharmaceutical industry and academia have developed many screening strategies to identify novel ligands that can modulate the activity of specific GPCRs. These compounds can be used as lead compounds for drug development.
Binding-based, stability-based, and cell signaling-based assays are developed to assess protein-ligand interactions. These assays are tailored to identify specific GPCR-targeted ligands. The physical interactions between a purified recombinant form of GPCR protein and test compounds are monitored using binding-based assays.
Cell signaling-based assays are used to determine downstream effectors of specific intracellular signaling pathways that are mediated by GPCR. The thermal stability of the purified test compounds is evaluated via stability-based assays. Fluorescence polarization and affinity mass spectrometry (MS) are the two most popular in vitro high throughput assays used for GPCR-targeted ligand screening.
The recent revolution in molecular modeling algorithms and protein engineering has accelerated research on GPCR. The discovery of a massive number of ligand-bound X-ray structures provided important insights into GPCR structure and function. Computational methods have been exploited to develop algorithms to simulate GPCRs in explicit lipid-water environments.5
Chemical libraries, such as ZINC and ChEMBL, offer the chemical diversity of screened potential GPCR ligands. For instance, the in-silico docking technique has been used to screen 138 million chemicals against the D4 dopamine receptor, the pharmacological target for schizophrenia.
Challenges in Developing GPCR-targeted Drugs
Most GPCR drugs have been developed based on high throughput screening (HTS) campaigns from large compound libraries. However, the compounds used for screening are not diverse enough and lack the features required to modulate the intended GPCR target.
Compared to other target families, the success rate of conventional HTS to GPCRs is low due to the dynamic structural features of GPCRs. Also, structural studies of GPCRs are associated with extensive resource-demanding purification of native receptors, which could be a challenging requirement for many laboratories.
GPCRs are inherently unstable when extracted from the lipid matrix. Also, these are flexible molecules that can assume distinct conformations. Hence, these would require structural stabilization before being used for therapeutic purposes. Multiple approaches have been explored to stabilize GPCRs in distinct conformations and to understand their pharmacology. For instance, camelid-derived immunoglobulin single variable domains (VHHs) were found to effectively stabilize disease-relevant pharmacological states and GPCR: signal transducer complexes. Therefore, VHHs can be used to positively accelerate drug discovery.
What are the Success Rates for GPCR-targeted Drugs?
It must be noted that the majority of GPCR-based drugs that are clinically used have been developed by exploiting only 30% of the identified GPCR genes. Therefore, there is an immense opportunity to develop new GPCRs-targeted drugs against various diseases.6
Around 40% of the commercially available drugs modulate GPCRs. GPCR-targeted drugs have exhibited significant effectiveness across a wide range of diseases, such as allergic rhinitis, hypertension, and schizophrenia.
Presently, many novel GPCR targets that have not yet been modulated by approved drugs are being evaluated in clinical trials. Of these, many peptide/protein-activated GPCRs are being assessed for their efficacy against varied diseases, such as the calcitonin-gene-related peptide (CGRP) receptor for migraine treatment, the GPR55 receptor for epilepsy, and the apelin receptor for cardiovascular disorders.
The US Food and Drug Administration (FDA) estimated the success rates for Phase I to be 70%, Phase II to be 33%, and Phase III to be 25-30% for all GPCR-target families. These estimates indicated more failures at later phases. For instance, the small-molecule drug fasiglifam, an antagonist of GPR40, exhibited significant effectiveness for diabetes but was terminated due to hepatotoxicity.7
Examples of the biotech/pharma companies involved with developing GPCR-targeted drugs are Structure Therapeutics, Septerna, and Escient Pharmaceuticals.8,9 At present, two GPCR drugs developed by Structure Therapeutics are in Phase I clinical trials for diabetes and obesity. Johnson & Johnson is also developing a GPCR-targeted drug for multiple myeloma.10
Sources
- Sriram K, and Insel PA. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol.2018; 93(4), 251-258. https://doi.org/10.1124/mol.117.111062
- Wise A, Jupe S.C, and Rees S. The identification of ligands at orphan G-protein coupled receptors. Ann. Rev. Pharmacol. Toxicol. 2004; 44:43–66.
- Hill S J. G-protein-coupled receptors: Past, present and future. British Journal of Pharmacology.2006; 147(1), S27. https://doi.org/10.1038/sj.bjp.0706455
- Smith J S, Lefkowitz R J, and Rajagopal S. Biased Signalling: from Simple Switches to Allosteric Microprocessors. Nat. Rev. Drug Discov. 2018; 17, 243–260. doi:10.1038/nrd.2017.229
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth, H B, and Gloriam DE. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017; 16(12), 829. https://doi.org/10.1038/nrd.2017.178
- Vassilatis DK, Hohmann JG, Zeng H. et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci. 2003;100(8):4903–4908. DOI:10.1073/pnas.0230374100
- Ciancetta, A. et al. Advances in Computational Techniques to Study GPCR-Ligand Recognition. Trends Pharmacol. Sci. 2015;36(12).
- Bell, J. Touting a new way to drug an all-important family of proteins, a startup launches with $100M. www.biopharmadive.com/.../. 2022; Assessed on December 20, 2023.
- Targeted Therapeutics for
Neurosensory-Inflammatory Disorders. Escient Pharmaceuticals. https://www.escientpharma.com/programs/pipeline/. 2023; Assessed on December 27, 2023.
- J&J’s next myeloma drug, Argenx’s second act and a new question for Bluebird: 3 ASH takeaways. www.biopharmadive.com/.../. 2023; Assessed on December 25, 2023.
Further Reading
Last Updated: Jan 11, 2024