Scientists have performed a new study that shows how the immune system has a damaging effect on a severe brain condition, which is most commonly caused by the cold sore virus.
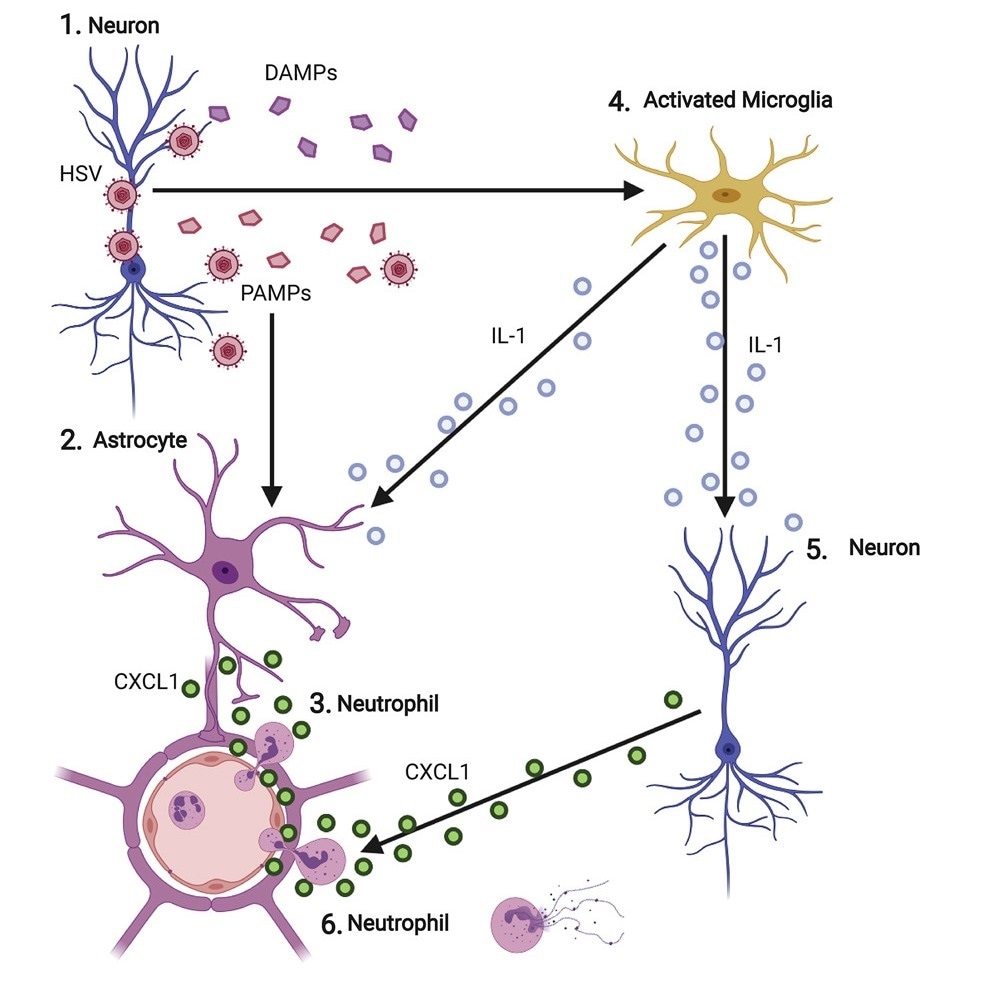
Graphical Abstract for “Astrocyte- and Neuron-Derived CXCL1 Drives Neutrophil Transmigration and Blood-Brain Barrier Permeability in Viral Encephalitis.” Image Credit: Michael BD, et al. Cell Reports 2020.
The researchers have successfully discovered a particular type of immune cell that causes brain inflammation in herpes simplex virus (HSV) encephalitis. Most importantly, the team has also established the signaling protein that recruits this immune cell into the brain from the bloodstream.
Published in the Cell Reports journal, the study results could lead to the development of targeted therapies for the brain infection—the major cause of viral encephalitis around the world.
HSV encephalitis is known to take hold rapidly and, in spite of rapid anti-viral drug therapy, several patients succumb to this infection. A majority of the survivors also suffer from brain injury because of the damage and inflammation caused by the HSV virus and by immune cells that gain access to the brain, disintegrating the blood-brain barrier.
Determining the roles of specific immune cells and the factors that allow them to cross the protective blood-brain barrier is critical to develop targeted immune-therapies.”
Dr Benedict Michael, Study Lead and Senior Clinician Scientist Fellow, University of Liverpool
With the help of a mouse model, the team demonstrated that neutrophils—a type of immune cell—rendered the blood-brain barrier more permeable and played a role in the brain damage linked to HSV encephalitis. The researchers also learned that these neutrophils were not required to manage the virus.
In the meantime, monocyte immune cells were identified to play a protective role and were required to regulate the virus and avoid damage to the brain.
The team also discovered the exact signaling protein—known as CXCL1—that caused these damaging neutrophils to migrate into the brain during HSV infection. By inhibiting this CXCL1 protein, neutrophils were stopped from crossing the blood-brain barrier and causing inflammation that leads to a less severe case of disease.
These discoveries make the CXCL1 protein an exciting target for novel treatments that can prevent the influx of damaging white blood cells without restricting the function of the protective ones.
There is currently no licensed treatment for the severe brain swelling which occurs despite antiviral therapy in HSV encephalitis. Sometimes steroids are given, but as these suppress the immune system in a very broad way, there is a risk of uncontrolled viral infection. There is an urgent need for targeted treatment that prevents damaging immune cells from entering the brain without limiting the immune cells needed to control the virus.”
Dr Benedict Michael, Study Lead and Senior Clinician Scientist Fellow, University of Liverpool
At present, Dr Michael and collaborators are planning to analyze the effect of the CXCL1 signaling protein in patients who earlier received steroids as part of a clinical trial headed by Professor Tom Solomon at the University of Liverpool.
Source:
Journal reference:
Michael, B. D., et al. (2020) Astrocyte- and neuron-derived CXCL1 drives neutrophil transmigration and blood-brain barrier permeability in viral encephalitis. Cell Reports. doi.org/10.1016/j.celrep.2020.108150.