Coronaviruses, similar to the one that causes COVID-19 infection, is embedded with protein “spikes” that attach to receptors on their victims’ cells—the initial step in infection.
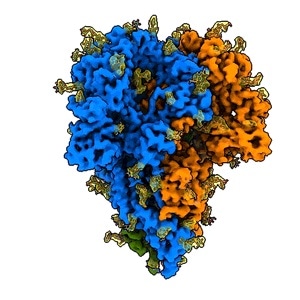
This rotating image shows the detailed structure of a “spike” from a coronavirus that causes cold symptoms – a milder relative of the virus that causes COVID-19. Spikes bind to receptors on the cells of their victims to start infections. They consist of three protein molecules, shown in blue, green, and orange, studded with sugar molecules (yellow) that play an important role in the virus’s life cycle and in its ability to evade the immune system. This image was made with a new method that shows spikes in their natural state, yielding quicker and more realistic snapshots of the infection apparatus. Video Credit: K. Zhang et al., Quarterly Reviews of Biophysics Discovery, 2020.
Researchers have now created the first comprehensive pictures of these protein spikes in their natural form, while still bound to the virus and without making use of chemical fixatives that may alter their shape.
According to the team, their new approach, which integrates computation and cryogenic electron microscopy (cryo-EM), should yield faster and more accurate images of the infection apparatus in multiple strains of coronavirus. This is a crucial step in developing therapeutic vaccines and drugs.
The advantage of doing it this way is that when you purify a spike protein and study it in isolation, you lose important biological context: How does it look in an intact virus particle? It could possibly have a different structure there.”
Wah Chiu, Study Senior Author and Professor, SLAC National Accelerator Laboratory, Department of Energy
Chiu is also a professor at Stanford University. The researchers have presented their findings in the Quarterly Reviews Biophysics Discovery journal.
It is known that seven strains of coronaviruses infect human beings. Among these, four strains cause comparatively mild illnesses, while the other three—such as SARS-CoV-2, the virus responsible for causing COVID-19—can be lethal, stated Jing Jin, the co-author of the study and an expert in the molecular biology of viruses at Vitalant Research Institute in San Francisco.
The Vitalant team generally searches for viruses in stool and blood samples collected from animals and humans, screens blood samples at the time of outbreaks, such as the present pandemic, and investigates the interactions that occur between viruses and their hosts.
According to Jin, the COVID-19 virus is so virulent that there are just a few cryo-EM laboratories in the world that can investigate it with a sufficient degree of biosafety controls.
Hence, for this analysis, the team studied a relatively milder coronavirus strain, known as NL63, which is responsible for causing common cold symptoms and accounts for around 10% of human respiratory disease every year. It is believed that this virus binds to the same receptors on the human cell surfaces as the COVID-19 virus does.
Instead of chemically extracting and purifying the spike proteins of the NL63, the team flash-frozen the entire, intact viruses into a glassy state that maintains the natural structure of their constituents.
They subsequently created scores of comprehensive images of arbitrarily oriented viruses by using cryo-EM instruments installed at the Stanford-SLAC Cryo-EM Facilities, then digitally acquired the bits containing the spike proteins, and finally integrated them to obtain high-resolution images.
The structure we saw had exactly the same structure as it does on the virus surface, free of chemical artifacts. This had not been done before.”
Jing Jin, Study Co-Author, Vitalant Research Institute
Furthermore, the researchers identified the sites where sugar molecules bind to the spike protein in a process known as glycosylation, which has a significant role to play in the life cycle of the virus and in its potential to escape the immune system. The researchers’ map contained three glycosylation sites that had been estimated but never directly observed before.
According to Jin, while a German team has employed an analogous technique to digitally obtain the spike protein images from the SARS-CoV-2 virus, initially they had to fix the virus in formaldehyde so that there would be no risk of this pathogen infecting others, and this treatment may induce chemical changes that interfere with observing the true structure.
Looking ahead, Jin added that the researchers would like to know how the portion of the spike that attaches to human cell receptors is stimulated, and also apply the same method to examine spike proteins from the COVID-19 virus, which would need dedicated biohazard containment facilities.
Source:
Journal reference:
Zhang, K., et al. (2020) A 3.4-Å cryo-electron microscopy structure of the human coronavirus spike trimer computationally derived from vitrified NL63 virus particles .Quarterly Review of Biophysics (QRB) Discovery. doi.org/10.1017/qrd.2020.16.