Research teams from the University of Helsinki have revealed how the motor protein, called myosin, which is responsible for causing the contraction of skeletal muscles, also functions in non-muscle cells to form contractile structures on the interior face of the cell membrane.
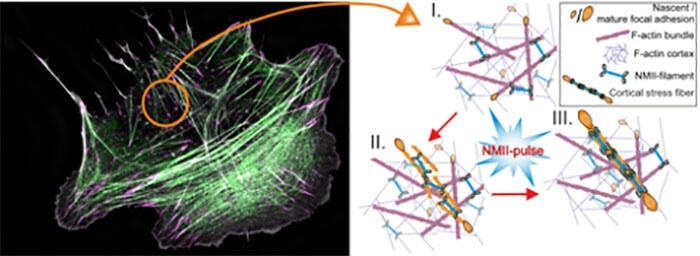
On the left: Super-resolution microscopy image of a migrating human osteosarcoma cell. Magenta marks focal adhesions, and green non-muscle myosin II (NMII). F-actin bundles are shown in grey. On the right: Schematics of cortical stress fiber assembly process. Myosin II pulse orchestrates the initial assembly and maturation of a cortical stress fiber, followed by focal adhesion maturation at the ends of the contractile bundle. Note that the color-coding is different between the two panels. Image Credit: Jaakko Lehtomäki.
This is the first time when such “mini-muscles,” also called stress fibers, have been observed to arise spontaneously via myosin-driven reorganization of the pre-existing actin filament network in cells. Any flaws in the assembly of these “mini-muscles” in cells can result in various diseases in humans and, in the most extreme cases, in cancer progression.
A recent research work, published in the eLife journal, investigates the central mechanisms of stress fiber assembly and shows how stress fibers can be directly built at the cortex of the cell; the cortex is a specialized network of actin filaments found on the internal face of the cell membrane.
The study, conducted in the teams of Academy Professor Pekka Lappalainen at the HiLIFE Institute of Biotechnology and Docent Sari Tojkander from the Faculty of Veterinary Medicine at the University of Helsinki, has revealed that myosin pulses, which were earlier associated with shape-changes in epithelial tissues at the time of animal development, can template the stress fiber assembly in the cell cortex.
In this mechanism, non-muscle myosin II, which is closely associated with the protein responsible for causing muscle contraction, is temporally and locally recruited to the cortex, in which it initially assembles the mesh-like actin filament network into parallel rod-like structures.
Such structures later stimulate the growth and maturation of focal adhesions at the two ends of the actomyosin bundle, eventually generating a stress fiber at the cell cortex, as shown in the above image.
“Previous studies from our group at the University of Helsinki and other laboratories abroad demonstrated that stress fibers can arise at the front of the cell from small actin- and myosin-containing precursor structures, and that stress fibers disassemble at the back of the cell as it moves forward. Now we reveal a completely new mechanism by which stress fibers can form in cells and provide an explanation for why 'mysterious' myosin pulses occur at the cell cortex,” commented Lappalainen.
Intriguingly, we also observed that this type of stress fiber generation was most prominent under the nucleus, which stores all genetic information and is the largest organelle in our cells. It could be that cortical stress fibers protect the nucleus or aid the movement of the nucleus along with the rest of the cell body.”
Dr Jaakko Lehtimaki, Study Lead Author, University of Helsinki
These new findings add a significant new aspect to the stress fiber toolbox. Cells in the 3D tissue environment seldom show stress fiber precursors that are usually seen in cells moving on a cell culture dish.
Thus, the assembly process mediated by myosin pulses allows the organization of contractile structures in cells migrating in different conditions. Since myosin pulses have been seen in several different types of cells and tissues, this may act as a universal process for producing local force in non-muscle tissues.
Source:
Journal reference:
Lehtimäki, J. I., et al. (2021) Generation of stress fibers through myosin-driven reorganization of the actin cortex. eLife. doi.org/10.7554/eLife.60710.