Scientists from Japan and the United States discovered that SARS-CoV-2 can knock out a significant molecular pathway associated with an immune complex named MHC class I. This observation could help researchers better comprehend how COVID-19 infection takes hold.
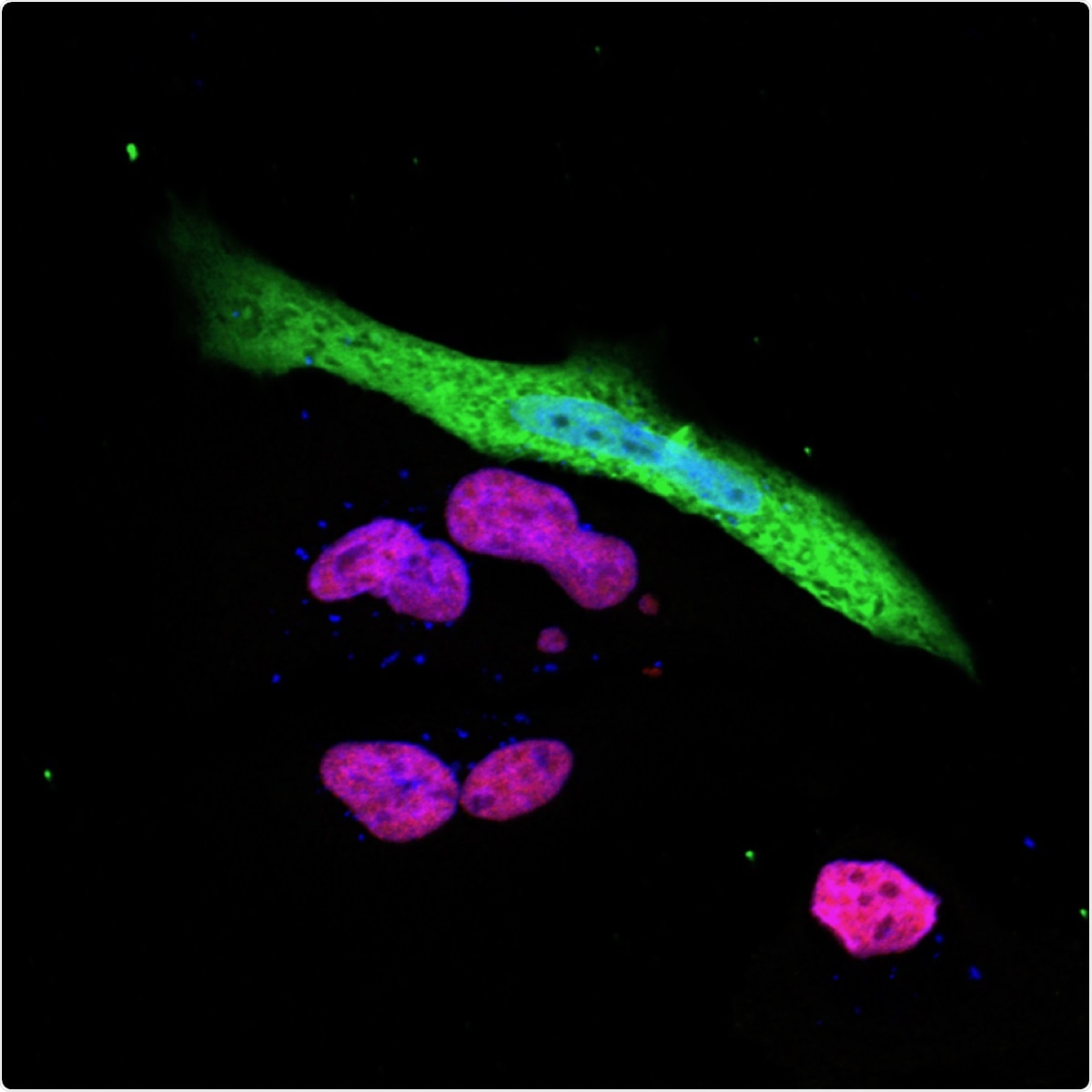
The expression of the immune response gene NLRC5 (red purple) is suppressed in SARS-CoV-2 (green) infected cells. Image Credit: Koichi Kobayashi.
Our discovery reveals how the virus can evade the human immune defense system and might help to explain why the pandemic has been so severe. The mechanisms we identify may provide new molecular targets for drug discovery.”
Koichi Kobayashi, Immunologist, Hokkaido University
Koichi Kobayashi headed the research and is also an immunologist at the Texas A&M University.
The researchers employed a bioinformatics approach to analyze how SARS-CoV-2—the virus that causes COVID-19—alters gene expression in the immune systems of COVID-19 patients when compared to uninfected people. This is a beneficial means to analyze the function of complicated cell signaling pathways that induce immune responses to combat harmful viruses and bacteria.
MHC (major histocompatibility complex) class I molecules are the key weapon in the immune response against viruses. Upon infection by a virus, the cell enables the expression of viral antigens on the infected cell surface, garnering the attention of immune cells named cytotoxic T cells. These immune cells annihilate the infected cells, along with the invading virus within them.
Along with examining gene expression in COVID-19 patients, the scientists also infected human cell lines with the SARS-CoV-2 virus to evaluate their observations.
The findings revealed that a protein from the SARS-CoV-2 virus, known as ORF 6, inhibits a host cell protein, known as NLRC5, accountable for activating the MHC class I pathway.
The research reveals that this occurs in two ways. ORF6 destructs cell signaling, thereby turning off NLRC5 expression. ORF6 also hinders the action of NLRC5.
Other infectious viruses like MERS and HIV also focus on the MHC class I pathway. The scientists presume that SARS-CoV-2 too targeted the pathway, however, this research was the first to reveal the mechanism.
Without the activation of the MHC class I pathway, viruses in the infected cells are essentially hidden from the immune system. That helps to explain why SARS-CoV-2 virus persists in the body and why it keeps infecting others, leading to the pandemic.”
Koichi Kobayashi, Immunologist, Hokkaido University
Further investigation would help in the identification and testing of drugs that inhibit the activity of the ORF6 viral protein, to reinstate host cells' capability to activate the major histocompatibility complex. If successful, these drugs would encourage the host immune system to annihilate the virus itself, efficiently enhancing immune responses.
Source:
Journal reference:
Yoo, J.-S., et al. (2021) SARS-CoV-2 inhibits induction of the MHC class I pathway by targeting the STAT1-IRF1-NLRC5 axis. Nature Communications. doi.org/10.1038/s41467-021-26910-8.