In this interview, we speak to Professor Charles Easley about his latest research into male infertility, and how sperm cells could be potentially developed from primate stem cells.
Please can you tell us about your background in health sciences and what inspired your latest research into sperm cells?
I obtained my BS in Biology from the College of William and Mary, my MS in Biology from Virginia Commonwealth University, and my Ph.D. in Biochemistry from Virginia Commonwealth University School of Medicine under the tutelage of Dr. Robert Tombes.
After completing my terminal degree, I was a postdoctoral fellow in OB/GYN and Reproductive Sciences in Dr. Gerald Schatten’s laboratory at the University of Pittsburgh School of Medicine. During my postdoctoral fellowship, I became very interested in patient-specific pluripotent stem cells and regenerative medicine.
In collaboration with Dr. Kyle Orwig, I became interested in whether we could derive functional spermatogenic cells from embryonic and induced pluripotent stem cells. In my postdoctoral fellowship working with Drs. Schatten and Orwig, I learned about the growing prevalence of male factor infertility and how limited the treatment options were for men with infertility in cases where they did not produce any gametes useful for in vitro fertilization.
What really stuck with me and truly inspired my research was learning about prepubescent male patients who were undergoing chemotherapy or full-body irradiation where there was a decent chance that the patients would be rendered sterile from their cancer treatments. I thought about how those poor kids had been robbed of their childhoods as they underwent treatments and when they reached adulthood, they had a chance of being infertile without any treatment options. One of my lab’s main goals has been to develop stem cell-based therapies to treat male factor infertility ever since.
It is estimated that around 9% of men in the US have experienced fertility problems and this statistic is on the rise. Why causes male infertility and why is the number of men suffering from this rising?
That’s a really interesting question, and the short answer is that we really don’t have a well-defined answer. Shanna Swan, in her book Countdown, highlights some of the potential causes including lifestyle and exposure to environmental toxicants.
Certainly, we’ve gotten better at diagnosing male infertility, and there are more discussions educating patients regarding the risk of infertility when undergoing cancer treatment thanks to the Oncofertility Consortium, but I think a gene-environment interaction really lies at the heart of the evergrowing population of infertile men.

Image Credit: Image Point Fr/Shutterstock.com
In your new research, you made sperm cells using primate embryonic stem cells. Can you describe how you carried out this research?
Absolutely. We originally developed a method for differentiating human embryonic and induced pluripotent stem cells into advanced spermatogenic cells including spermatogonia-like cells, primary spermatocyte-like cells, secondary spermatocyte-like cells, and round spermatid-like cells.
For ethical and legal reasons, we could not assess whether spermatid-like cells were functional and could fertilize an egg. So, we used non-human primate (rhesus macaque) embryonic stem cells for this recent study because we could assess whether our in vitro spermatid-like cells could fertilize a non-human primate egg.
Just as in our previous publication with human pluripotent stem cells, we coaxed non-human primate embryonic stem cells into advanced spermatogenic cells, including round spermatid-like cells. We took our round spermatid-like cells and injected them into non-human primate oocytes (eggs) and examined the ability of the spermatid-like cells to fertilize the oocytes and develop to the blastocyst stage.
What did you discover?
We discovered that like human embryonic and induced pluripotent stem cells, non-human primate pluripotent stem cells could differentiate into advanced spermatogenic cells such as spermatogonia-like cells, primary spermatocyte-like cells, secondary spermatocyte-like cells, and round spermatid-like cells. Round spermatid-like cells could fertilize non-human primate oocytes and successfully develop to the blastocyst stage in vitro but like in vivo round spermatids, our in vitro-derived spermatid-like cells were immature and required oocyte activation to undergo development.
Additionally, we discovered that round spermatid-like cells required the addition of TET3, an important enzyme that is required for rapid de-methylation of the male pronucleus after fertilization. Mature sperm carry TET3, but round spermatids do not express high levels of the enzyme. We discovered that we could inject purified TET3 at the same time that we injected in vitro-derived round spermatid-like cells to improve fertilization and developmental success rates.
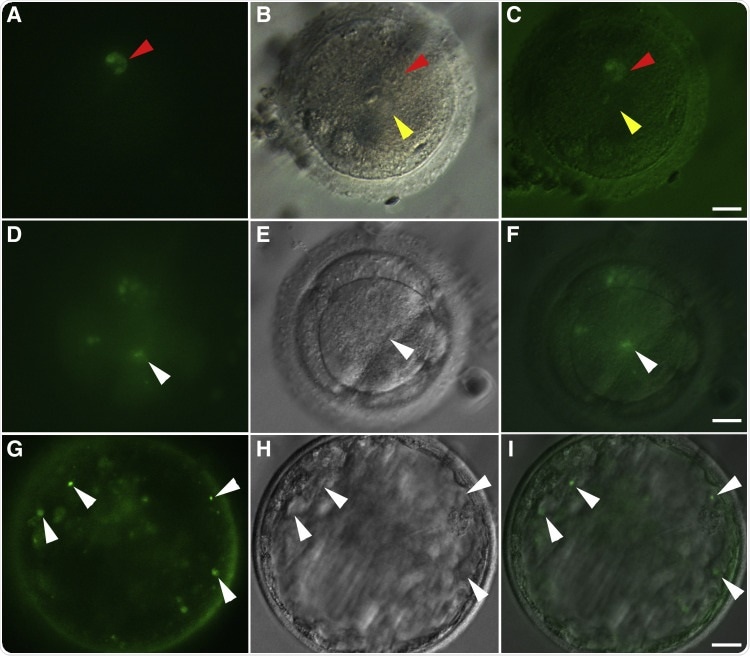
Image Credit: DOI: https://doi.org/10.1016/j.xfss.2021.09.001
Previous research has been able to produce sperm-like cells using mouse stem cells but their sperm production is distinctly different to humans. How is this?
I believe you are referencing Dr. Mitinori Saitou’s outstanding work. Yes, his lab was the first to generate functional sperm-like cells using a tissue explant approach after transplantation of primordial germ cell-like cells derived from mouse pluripotent stem cells. This work was absolutely groundbreaking, but there are some biological and kinetic differences in rodent spermatogenesis compared to human and non-human primate spermatogenesis that may make this work harder to translate to humans.
For instance, primordial germ cell transplantation into testes leading to restoration of spermatogenesis in sterile testes has not been successful in several animal models including non-human primates.
While there are many similarities between rodents and human/non-human primate spermatogenesis, rodents have a smaller spermatogonial stem cell pool, have more transit-amplifying events, replace a larger percentage of their histones with protamines, and do not require paternal/sperm centrioles for the first cell division after fertilization. These differences necessitated using a species more similar to humans if we ever hope to develop these technologies in the clinic to treat infertile male patients.
You used rhesus macaque monkeys as a model for your latest research. Why did you choose this primate in particular?
Similar to your last question, we chose non-human primates (rhesus macaque) because this animal model is more similar to humans in terms of spermatogenesis and fertilization.
It’s not exact, non-human primates do have an extra amplifying event that humans do not, but overall it is a more-closely related model to us.

Image Credit: 700 Pixel/Shutterstock.com
How will your research provide hope for future clinical therapies?
Realistically, we are a long way off from the clinic. While we were able to produce healthy non-human primate embryos in our current study, we need to show that we can produce healthy non-human primate offspring from those embryos and demonstrate that offspring derived from in vitro spermatids do not have any developmental or aging issues.
We are currently working on this goal, but until we confirm that we can produce healthy offspring, we really cannot attempt to move this work to the clinic.
What are the next steps for your research?
We next are examining whether we can produce healthy offspring (non-human primates). Additionally, we are improving our in vitro differentiation protocol to better simulate a testis environment in a dish.
Our hope is that by improving our differentiation protocol, we can make more advanced spermatid-like cells to improve fertilization and developmental success rates.
Where can readers find more information?
About Professor Charles Easley
Dr. Charles Easley is an Associate Professor and Graduate Program Coordinator in the Department of Environmental Health Science in the College of Public Health at the University of Georgia. Dr. Easley has served as Chair of the Regenerative Medicine and Stem Cell Biology Special Interest Group within the American Society for Reproductive Medicine.
Additionally, Dr. Easley has served on the Executive Committee for Public Policy within the American Society for Cell Biology to help educate Members of Congress on the importance of healthy funding for the NIH and NSF. Work in the Easley Lab focuses on 3 major themes: 1) Impacts of Environmental Exposures on Human Spermatogenesis, 2) Regenerative Medicine, and 3) Drug Discovery.
The Easley lab primarily uses human pluripotent stem cells and our novel in vitro model of spermatogenesis to examine the effects of environmental exposures on spermatogenesis as well as identify potential male contraceptives. In our regenerative medicine portfolio, we use non-human primate pluripotent stem cells as a platform to begin deriving stem cell-based therapies towards novel treatments for male factor infertility. Dr. Easley’s lab is funded by grants from the National Institutes of Health, Bill and Melinda Gates Foundation, and the Georgia Research Alliance.