Reviewed by Danielle Ellis, B.Sc.Feb 14 2022
Researchers from the University of California San Francisco discovered two functional archetypes of metastatic cells spanning seven different types of brain cancers, each having both immune and non-immune cell types, using data from over 100,000 malignant and non-malignant cells from 15 human brain metastases.
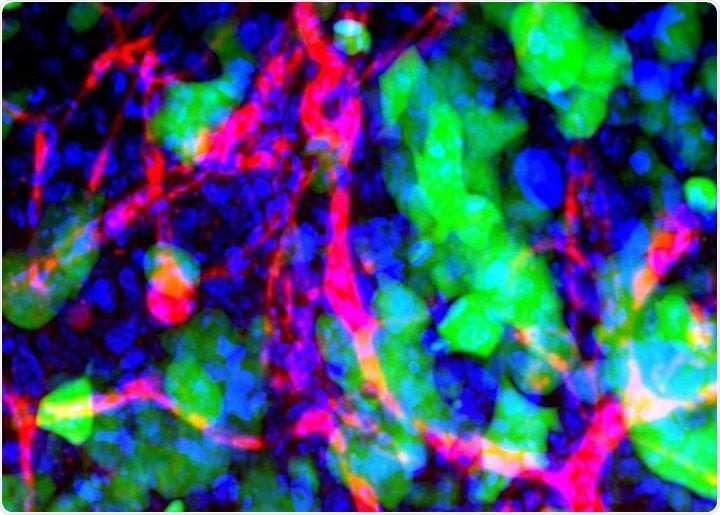
Microscopic image of metastasized cancer cells (green) in a mouse’s brain. Image Credit: Emily Wyatt, Mark Davis, California Institute of Technology.
Their findings may serve as a roadmap for the development of metastatic tumors, which might be used to develop medicines to enhance the treatment of metastasized patients. The study was published on February 17th, 2022, in the journal Cell.
The UCSF researchers investigated metastatic tumor cells (MTCs) and discovered eight functional processes expressed by MTCs across seven forms of metastatic brain cancer, led by first author Hugo Gonzalez, PhD, and senior authors Jeroen Roose, PhD, and the late Zena Werb, PhD.
These unique and complementary processes operate together within single cells to build two recurrent cell archetypes, one inflammatory and the other proliferative, that co-exist within each metastatic tumor and are both shaped by immune cells, according to the researchers.
The most prevalent type of brain cancer is brain metastasis, which happens roughly ten times more frequently than cancer that develops in the brain. While brain metastasis therapy options have improved significantly, there is still much to learn about metastasis genesis.
To determine and comprehend the recurrent patterns that characterize the mechanism of metastasis formation in patients, the researchers coupled high-dimensional single-cell analysis of human brain tissue metastases from various cancer types with experimental models.
They also discovered a similar metastatic niche or microenvironment, as well as an immunosuppressive stroma enriched with T-cells and metastasis-associated macrophages, which appear to be important in the dynamics of the two archetypes.
These archetypes co-exist within each metastatic tumor. For the MTCs that are not proliferating, these cells get reprogrammed to express genes for inflammation, stress, and other changing conditions. It’s likely that these tumor-immune interactions are shaping the state of the MTCs.”
Hugo Gonzalez PhD, Study First Author, University of California San Francisco
Inspiration from a renowned cancer researcher
Zena Werb was the first person that saw the potential and feasibility of collecting human metastases and combining them with cutting-edge technologies such as single-cell transcriptomics and CyTOF. She believed that by analyzing human brain metastases, we could determine the relationship between these cellular processes orchestrated by MTCs and their specific microenvironments.”
Hugo Gonzalez PhD, Study First Author, University of California San Francisco
Werb, a world-renowned cancer biologist and associate director for basic science from the University of California, San Francisco’s Helen Diller Family Extensive Cancer Center, revolutionized the field by emphasizing the importance of cells’ local “neighborhoods” in determining tumor growth and behavior.
Her work paved the way for immunotherapy and other current methods of cancer treatment over the span of four decades. Werb died in 2020 at the age of 75, but her legacy continues with her colleagues—who looked up to her as a mentor.
From the beginning, she believed in this project and encouraged me to persevere even when the collection and processing of these rare and small samples were quite difficult. Zena also helped orchestrate fruitful collaborations with UCSF colleagues Joanna Phillips, MD, PhD, and Matthew Spitzer, PhD, who were critical for this large project.”
Hugo Gonzalez PhD, Study First Author, University of California San Francisco
Gonzalez’s study, according to Roose, laid a solid foundation for the team’s collaboration with the UCSF Endeavor initiative, which aims to better understand how cancer cells interact with the host cells that encircle the tumor to cause metastases.
Roose has found it extremely worthwhile to see Hugo through the home stretch of this brain metastasis project. “I can just see Zena walk into my office, giving us a thumbs up and a big hug,” added Roose.
Source:
Journal reference:
Gonzalez, H., et al. (2022) Cellular architecture of human brain metastases. Cell. doi.org/10.1016/j.cell.2021.12.043.