Malaria is known as a disastrous disease, with 247 million cases and 619,000 deaths reported in 2021 alone.
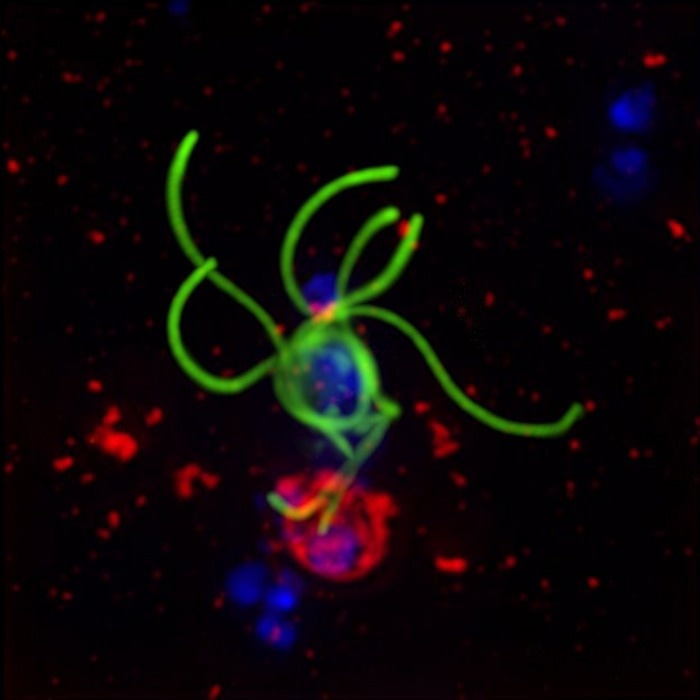 The human-infecting malaria parasite, Plasmodium falciparum (green), is depicted erupting from human red blood cells (red). Eight sexually mature parasites (green) emerge from the human cell (red), with their replicating DNA shown in blue. Image Credit: Dr Sabrina Yahiya and Professor Jake Baum.
The human-infecting malaria parasite, Plasmodium falciparum (green), is depicted erupting from human red blood cells (red). Eight sexually mature parasites (green) emerge from the human cell (red), with their replicating DNA shown in blue. Image Credit: Dr Sabrina Yahiya and Professor Jake Baum.
Malaria causes fever and a flu-like illness that happens when people are infected with the parasite Plasmodium falciparum, which is spread by mosquitoes. In the past few years, drugs to handle malaria symptoms and insecticides to kill malaria-spreading mosquitoes have enhanced. However, the parasite and the mosquitoes are growing resistant to such strategies.
Hence, there is an immediate necessity for new antimalarial drugs, and the main aim is to avoid parasite spread by hindering their passage from human to mosquito, this is something that relies on the sexual phase of the parasite life cycle.
The Baum laboratory together with collaborators at Imperial College London, UK, earlier determined a new kind of potent antimalarial compound, belonging to a class of sulfonamides.
Such compounds tend to kill the parasite only when it is in a particular sexual phase of its life cycle. This is quickly halting it from being capable to infect a mosquito and, thus, avoiding any consequent human infection.
In their new Disease Models & Mechanisms article, Baum and collaborators examined exactly how such compounds work, which is a necessary step before the compounds could be advanced for testing in patients.
Targeting parasite transmission from human to mosquito and back again is pivotal if we hope to reach the goal of worldwide malaria elimination. If you only treat one symptomatic patient, you address their symptoms but neglect the issue of malaria spread. By limiting transmission, however, you can radically curtail the spread of malaria across a population.”
Dr Sabrina Yahiya, Study Lead Author, Kymab Ltd
The research group started by growing human red blood cells infected with the malaria parasite in the laboratory, and further manipulating the parasites to go into their sexual life stage.
Further, the scientists treated these parasites with one of the sulfonamide compounds to determine which parasite proteins were being targeted through the transmission-blocking compounds.
Consequently, the researchers employed “click chemistry,” a method that won the 2022 Nobel Prize in Chemistry to fix a chemical label to the sulfonamide compounds.
Further, this label would tag any parasite proteins that came in contact with them. This method determined a parasitic protein known as Pfs16 as developing a powerful bond with the drug.
Fascinatingly, Pfs16 is significant for the sexual conversion of the malaria parasite. Further, the team executed extra experiments to verify that the sulfonamides bind Pfs16 and, significantly, block its function.
Further, the researchers wished to pin down the exact point in the sexual phase of the parasites, which were being targeted by the sulfonamides. Once the malaria parasites commit to either male or female forms in human blood, they could be sent to mosquitoes and once in the mosquito gut develop into a highly mature sexual phase.
These mature male and female parasites—like human egg and sperm—then fuse to allow sexual reproduction. The newly reproduced parasites experience additional maturation and are further transferred by the mosquito to infect more humans.
The process of sexual maturation, which generally happens in the mosquito gut, could be activated in an artificial manner in the laboratory and takes around 10 to 25 minutes completely.
Particularly, the authors discovered that the sulfonamide compounds targeted male parasites and uniquely hindered their sexual maturation if administered to the parasite within the first 6 minutes of the sexual maturation process. This is the same time that the parasitic protein target, Pfs16, plays a significant role in hindering male parasite maturation.
By determining the compound’s target and window of activity, this work offers a highly accurate knowledge of the parasites’ life cycle stage during which this class of sulfonamides was in an effective state. Also, it stresses the special potential of such compounds to quickly block sexual maturation, and by extension, malaria parasite transmission, by targeting the significant parasite protein, Pfs16.
On the whole, Baum and collaborators have determined how this new class of antimalarials blocks the parasite from reaching sexual maturity, and hence, their spread from human to human through a mosquito bite.
This is a significant step in developing efficient new drugs to decrease the huge number of new malaria cases throughout the world. Once completely developed and tested, it is possible to give these compounds to patients with malaria together with current therapies for treating their symptoms and to avoid the parasite from being spread to more individuals.
The unique ability of this class of sulfonamides to potently block sexual maturation of the parasite with almost immediate impact makes the direct delivery of the compounds to the mosquito a very appealing alternative administration strategy.”
Jake Baum, Professor, University of New South Wales
It is possible to achieve this exciting alternative strategy by coating sugar baits or mosquito nets with the compounds. More research is in progress to explore and improve the activity of this class of sulfonamides for making use of either in humans or directly with mosquitoes.
However, this study expands the breadth of strategies available to utilize in the fight against malaria.
Source:
Journal reference:
Yahiya, S., et al. (2023) A novel class of sulphonamides potently block malaria transmission by targeting a Plasmodium vacuole membrane protein. Disease Models & Mechanisms. doi.org/10.1242/dmm.049950