Reviewed by Danielle Ellis, B.Sc.Dec 5 2023
An examination investigating the impact of hypochlorous acid (HOCl), commonly recognized as bleach, produced during a cell’s immune response (phagocytosis) combating the common fungal pathogen Candida albicans, reveals the potent lethality of HOCl.
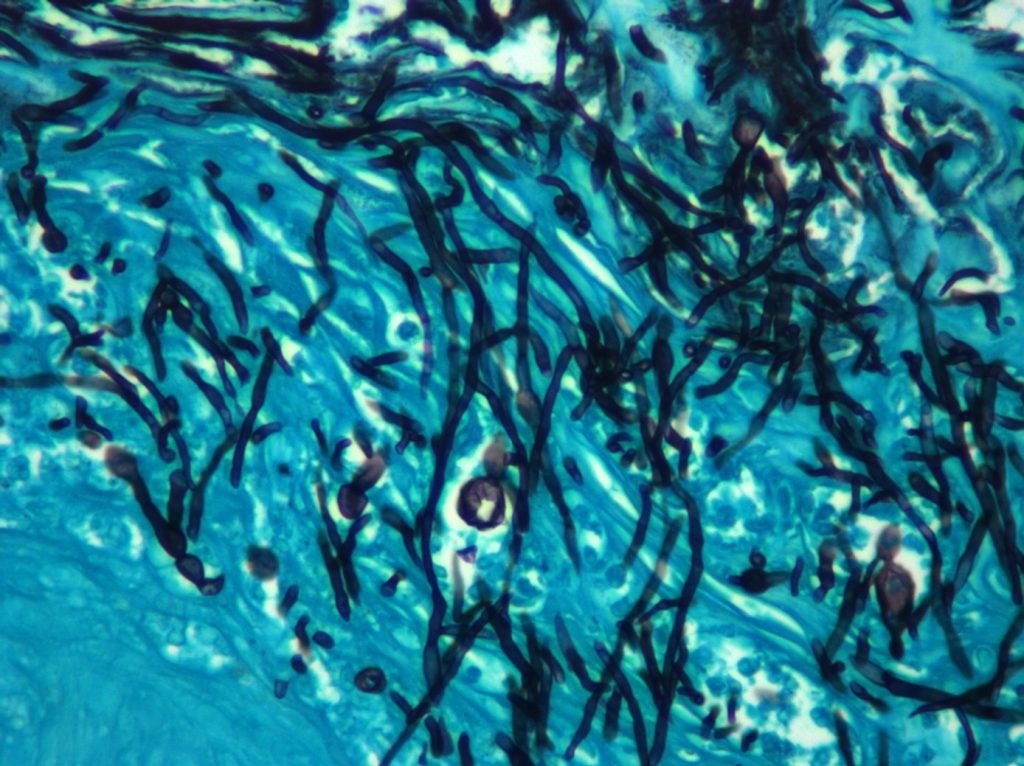 These are C. albicans cells growing invasively into tissue in a mouse model of an oral infection. The candida hyphae are stained black, and the tissue is stained a blue/green. Image Credit: James Konopka.
These are C. albicans cells growing invasively into tissue in a mouse model of an oral infection. The candida hyphae are stained black, and the tissue is stained a blue/green. Image Credit: James Konopka.
This laboratory discovery, highlighted in an upcoming issue of the American Society of Microbiology’s mBio, also sheds light on the mechanisms through which HOCl operates in the process of killing.
This research marks a significant stride towards considering HOCl as an innovative therapeutic approach against C. albicans and potentially other pathogens.
C. albicans pose a substantial global infection risk, particularly proving highly virulent in immunocompromised patients and serving as the culprit behind severe systemic infections in this demographic. While numerous treatments have proven effective against this fungal pathogen over the years, drug resistance has persistently presented challenges in managing C. albicans infections.
Previous studies examining the immune response to the fungal pathogen have primarily centered on hydrogen peroxide (H2O2) rather than HOCl. During the phagocytosis process, phagocytes capture the fungal invader, leading to the creation of two oxidants—H2O2 and HOCl.
Myeloperoxidase converts H2O2, generated during the oxidative burst in the phagosome, into HOCl, which emerges as the more potent agent for extermination.
We discovered that hypochlorous acid kills cells by targeting the plasma membrane and oxidizing cellular components in a very different way than hydrogen peroxide. It disrupts the C. albicans plasma membrane, produces a very different transcriptional response than hydrogen peroxide, is more effective and disruptive to the plasma membrane, and therefore has a more distinct effect on killing these fungal cells.”
James Konopka PhD, Study Lead Author and Professor, Department of Microbiology and Immunology, Renaissance School of Medicine, Stony Brook University
According to Konopka, neutrophils play a pivotal role in regulating infections caused by C. albicans and other fungal pathogens.
Their significance lies in their distinctiveness, as they produce elevated levels of myeloperoxidase compared to other phagocytes, such as macrophages. This research underscores the critical role of the neutrophil response, integral to the oxidative process that generates the potent antifungal agent HOCl or bleach.
Although the outcomes of the laboratory study may not yield immediate implications for novel treatments targeting C. albicans infections, Konopka posits that the discoveries establish a foundation for devising fresh therapeutic approaches against this globally pervasive pathogen.
Source:
Journal reference:
Douglas, L. M., et al. (2023) Candida albicans resistance to hypochlorous acid. mBio. doi.org/10.1128/mbio.02671-23