Learn about some of the different approaches in electrophoresis and how these analytical methods can be applied to biochemistry.
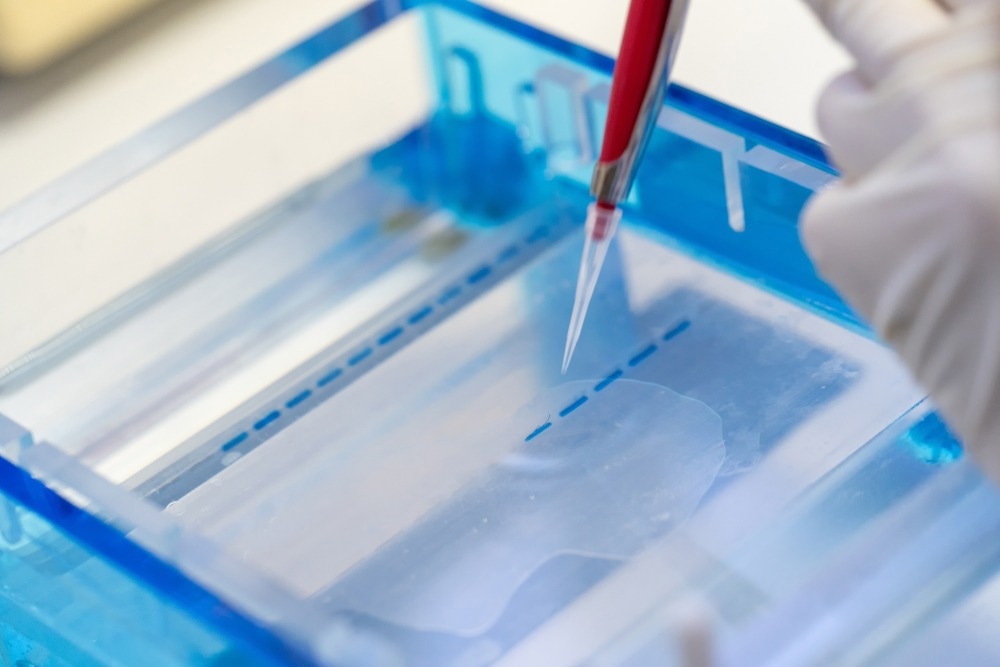
Image Credit: Choksawatdikorn/Shutterstock.com
What is Electrophoresis?
Electrophoresis is a simple, rapid, and sensitive analytical technique that is frequently used for both research and industrial applications for the separation of proteins, as well as nucleic acids, including ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), based on their shape and length. Importantly, in addition to proteins and nucleic acids, many other molecules can be separated through electrophoresis, some of which include cations, anions, amino acids, lipids, saccharides, peptides, nucleic acids, and even whole cells.
The separation of these macromolecules is primarily based on their charge; however, several other factors contribute to the mobility of these molecules through an electric field, which includes field and ionic strength, as well as the viscosity and pore size of the matrix through which the molecule migrates.
Proteins are considered ampholytes, as they consist of both positively and negatively charged particles. However, the weakly acidic isoelectric point of most proteins allows for their movement from the negatively charged cathode to the positively charged anode during electrophoresis for separation and quantification. Comparatively, the phosphate groups in both DNA and RNA allow these nucleic acids to easily migrate to the anode in a neutral or alkaline solution.
The porous nature of polyacrylamide and agarose gels has supported their routine use for electrophoresis applications. Whereas agarose gels have large pore sizes ideal for separating nucleic acids and large protein complexes, the smaller pore size of polyacrylamide gels makes this matrix ideal for separating smaller proteins and nucleic acids. Although agarose and polyacrylamide matrices are most frequently used for biochemical applications, paper, cellulose acetate, and starch gels can also be used for electrophoresis experiments.
Polyacrylamide Gel Electrophoresis (PAGE)
For most PAGE applications, the polyacrylamide gel, which has at least ten wells for samples, is vertically placed between two buffer chambers. An electrical field is subsequently applied across the buffer chambers, thereby facilitating the migration of proteins from the samples into and through the gel.
PAGE can be performed using continuous or discontinuous buffer systems. The continuous buffer system utilizes a buffer at a constant pH that can be shared between the gel, sample, and electrode reservoirs. Although proteins can be uniformly separated in continuous buffer systems, this approach often causes the resulting protein bands to be obscure and difficult to visualize, thus limiting their use for protein analyses.
Comparatively, a discontinuous buffer system utilizes two different buffers for the gels and electrode solutions, which allows proteins to migrate quickly through the large pore stacking gel before entering the lower small pore resolving gel, wherein proteins subsequently move slower. This buffer system is associated with superior resolution, thus allowing for its use for various biochemical applications.
Sodium Dodecyl Sulfate PAGE (SDS-PAGE)
Incorporating SDS into a discontinuous denaturing buffer system led to the development of SDS-PAGE, which remains the most common type of protein electrophoresis assays performed for biomedical studies. SDS is a negatively charged solution that denatures proteins by wrapping itself around the polypeptide backbone of these macromolecules.
When added to the buffer, SDS masks the intrinsic negative charge of proteins at a consistent charge-to-mass ratio of 1.4 grams of SDS for one gram of polypeptide for all proteins within a sample. In addition to high resolution and relative feasibility, SDS-PAGE only requires microgram quantities of protein for analysis, thereby reducing the unnecessary and excessive loss of samples associated with conventional electrophoresis techniques.

Image Credit: Gorodenkoff/Shutterstock.com
Applications of Electrophoresis in Biochemistry
Biochemistry involves examining chemical processes that occur within and relate to living organisms, thus making this area an essential aspect of medical and ecological research. Moreover, biochemistry can be further subdivided into structural biology, enzymology, and metabolism.
Drug Discovery
Within drug discovery, a thorough understanding of biochemical principles is essential in quickly and efficiently moving candidate drugs from the preclinical stages of development to therapeutics that can improve the quality of life for patients suffering from various diseases. To this end, electrophoresis is often incorporated into various preclinical assays to examine how novel pharmacological compounds interact with proteins of interest and whether their treatment leads to increased or reduced expression.
In addition to elucidating the direct effects of candidate drugs on target proteins, electrophoresis can also provide important information on how these agents impact the expression of other proteins that may be involved in the disease process. Likewise, electrophoresis can provide crucial insights into unwanted off-target effects and potential toxicity that could limit the applicability of test compounds for future in vivo studies and human trials.
Within molecular biology laboratories, agarose gel electrophoresis is often incorporated into polymerase chain reaction (PCR)-based genotyping analyses, as well as the production and screening of plasmids that may be used for protein production, gene expression analysis, and clustered regular interspaced short palindromic repeats (CRISPR).
For example, gel electrophoresis can be used to screen plasmids for their correctness, thereby allowing hundreds of colonies to be efficiently and rapidly screened while reducing the amount of sample needed for these crucial assays. One of the key advantages of gel electrophoresis in these applications is the ability of agarose gels to be reused, thus reducing the costs and time associated with performing these experiments.
Food Sciences
Capillary electrophoresis (CE) is associated with high separation efficiency, extremely small sample and reagent requirements, as well as rapid turnaround time for obtaining results. Within the food industry, CE is frequently used to detect and analyze a wide range of biomolecules, including carbohydrates, organic acids, flavonoids, pigments and colors, inorganic and organic compounds, vitamins, amino acids, proteins, and DNA, as well as the presence of bacteria, cells and viruses in food and beverage products.
Some of the most common food products that are subjected to CE include fruit, wine, tea, juice, beer, cereal, soft drinks, milk, vegetables, fish, meat, cheese, and eggs. In the dairy industry, for example, milk products are often subjected to heat treatments and drying, which can lead to protein denaturation and undesirable Maillard reactions that ultimately affect the nutrition and flavor profiles of products. Thus, CE is an important technique that is often used to quickly and quantitatively measure the levels of different proteins and peptides present in dairy products prior to their distribution to the public.
Sources:
Protein Electrophoresis Methods [Online]. Available from: www.bio-rad.com/.../protein-electrophoresis-methods?ID=LUSOW4GRI.
Overview of Protein Electrophoresis [Online]. Available from: www.thermofisher.com/.../overview-electrophoresis.html.
What is biochemistry? [Online]. Available from: https://www.ferrovial.com/en/stem/biochemistry/.
Asad, N., Smith, E., Shakya, S., et al. (2023). Sustainable Methodologies for Efficient Gel Electrophoresis and Streamlined Screening of Difficult Plasmids. Methods and Protocols 6(2). doi:10.3390/mps6020025.
Further Reading
Last Updated: Jan 8, 2024