Liposomes are spherical vesicles formed of a phospholipid bilayer that can be used to encapsulate and deliver a variety of drugs. They are created by artificially interrupting a phospholipid membrane, causing it to form a spherical shape to maintain hydrophilic interactions.
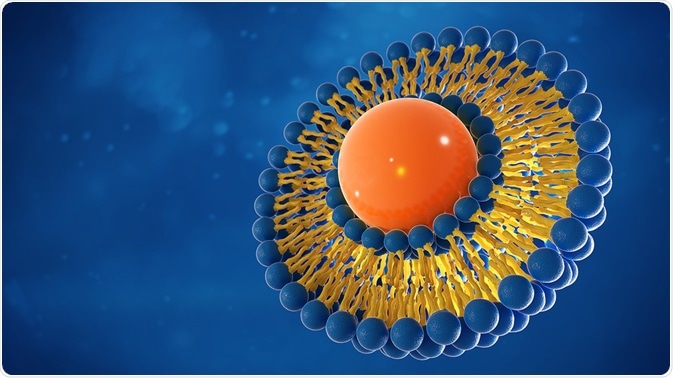
Image Credit: luminance studio/Shutterstock.com
Liposomes can carry molecules internally that are either hydrophobic or hydrophilic, as they will either interact with the bilayer or remain in the core of the sphere, respectively. Immunoliposomes are formed when antibodies or antibody fragments are conjugated to the surface of liposomes to allow targeted delivery of the liposome’s content.
In this manner, antibodies that show specificity to cancer-unique surface receptors can be used to deliver chemotherapeutic drugs in a way that limits the toxicity caused by traditional intravenous chemotherapy treatments.
Immunoliposomes
Liposomal drug delivery has previously relied upon the size of the liposome, with smaller liposomes being more likely to reach the intended tissue, due to the additional permeability of vasculature surrounding damaged and inflamed tissues. This limited targeting meant that drugs were frequently released from liposomes in other tissues, causing similar systemic toxicity to traditional intravenous chemotherapy treatment for cancer patients.
To increase the stability of liposomes and increase their half-life in the bloodstream, a polymer known as polyethylene glycol (PEG) is added. These liposomes are known as pegylated liposomes and are less susceptible to opsonization and therefore phagocytosis.
While pegylation increases the likelihood of liposomes reaching diseased tissue, it does not increase their specificity and there is still a large possibility for non-specific drug release. One solution to this issue is the addition of antibodies or antibody fragments, creating antigen-specific immunoliposomes.
Antibodies have previously been conjugated to the bilayer structure of liposomes, however, the addition of PEG surrounding the structure limits antibody availability and therefore binding capacity. To reduce this issue, antibodies and antibody fragments can be conjugated to the external end of PEG chains, ensuring they are exposed for binding.
The antibodies and fragments used in immunoliposome development are specific to surface antigens presented by certain cell types, allowing the immunoliposome to fuse directly to the target cells, releasing their drug contents in a controlled manner.
Drug release occurs internally, due to the natural endocytic action of cells following receptor binding. The immunoliposome and its contents are brought into the cell in a vesicle known as an endosome, where the liposome bilayer is digested and the contents released into the cytosol.
Antigen targeting allows disease-specific delivery of drugs due to the difference in expression levels of certain antigens on the cells within diseased tissues. In inflammatory diseases, endothelial cells present various adhesion molecules such as selectins and integrins to aid the process of extravasation.
These adhesion molecules, including intercellular adhesion molecule 1 (ICAM-1) can be targeted by specific antibodies, aiding in the treatment of conditions such as rheumatoid arthritis. This targeting leads to localized drug release with little toxicity due to the overexpression of ICAM-1 in arthritic sites, and its low levels of expression in healthy tissue.
Use of immunoliposomes in cancer therapy
While important for the development of treatments for inflammatory conditions, immunoliposomes are also a promising area of research in cancer therapy. Cancerous cells frequently express either novel antigens, known as neoantigens, or upregulated quantities of normal surface antigens, due to the genetic mutations that they possess. This means that these antigens can be used to target cancer cells with high specificity.
One example of an overexpressed protein in breast cancer is the human epidermal growth factor receptor 2 (HER2). This is a protein that is overexpressed in around 20% of breast cancer cases, making it a viable target for therapeutic research.
A humanized version of a murine monoclonal antibody, known as rhuMAb HER2 or trastuzumab, has been developed, which can specifically target the extracellular domain of HER2. Fragments of this anti-HER2 antibody conjugated to a liposome have been able to bind selectively to cancerous breast cells, delivering the immunoliposome’s encapsulated doxorubicin (a commonly used chemotherapy drug) without toxicity to non-mutated cells.
The use of immunoliposomes, in this case, is highly beneficial, as doxorubicin is very cytotoxic and leads to side effects such as hair loss, nausea, and cardiotoxicity when used intravenously.
HER2 is just one potential target for immunoliposomal targeting in cancer treatment. Additionally, clinical trials are studying the efficacy of targeting epidermal growth factor receptor (EGFR) in breast cancer, glioma, and colorectal cancer, again using doxorubicin as the encapsulated drug. The antigen-binding fragment (Fab) of an antibody known as Cetuximab has been used to deliver immunoliposomal drugs successfully to colorectal cancer cells.
Image Credit: StudioMolekuul/Shutterstock.com
Potential for immunoliposome treatments in the future
Immunoliposome treatment for cancer is not particularly widespread currently, mostly due to the cost of treatment development. However, due to significant correlations that have been shown between levels of antigen overexpression and successful drug targeting in cancerous tissues, immunoliposome drug delivery may become a more prevalent part of personalized cancer therapies.
Personalized cancer medicine can, overall, be more cost-effective due to factors such as length of treatment, reduction of recurrent cancers, and reduction of both occurrence and severity of side effects. This is because personalized treatments focus on the genetic mutations and expression of cancer-related proteins in biopsies of tumors, allowing treatments to be preferentially selected based on predicted efficacy.
There is also scope for the use of many other antibodies for immunoliposome targeting, such as those used in cancer immunotherapy, broadening the types and severities of cancers that could be treated in this way.
References
- Alavizadeh, S. H., Soltani, F. and Ramezani, M. (2016) ‘Recent Advances in Immunoliposome-Based Cancer Therapy.’ Current Pharmacology Reports.
- Bendas, G. (2001) ‘Immunoliposomes: A promising approach to targeting cancer therapy.’ BioDrugs.
- Green, A. E. and Rose, P. G. (2006) ‘Pegylated liposomal doxorubicin in ovarian cancer.’ International Journal of Nanomedicine.
- Hua, S. (2013) ‘Targeting sites of inflammation: Intercellular adhesion molecule-1 as a target for novel inflammatory therapies.’ Frontiers in Pharmacology.
- Jackson, S. E., and Chester, J. D. (2015) ‘Personalised cancer medicine.’ International Journal of Cancer.
- Merino, M., Zalba, S. and Garrido, M. J. (2018) ‘Immunoliposomes in clinical oncology: State of the art and future perspectives.’ Journal of Controlled Release.
- Park, J. W., Kirpotin, D. B., Hong, K., Shalaby, R., Shao, Y., Nielsen, U. B., Marks, J. D., Papahadjopoulos, D. and Benz, C. C. (2001) ‘Tumor targeting using anti-her2 immunoliposomes.’ In Journal of Controlled Release.
- Paszko, E. and Senge, M. O. (2012) ‘Immunoliposomes.’ Current Medicinal Chemistry.
Further Reading
Last Updated: Dec 7, 2020