DNA strand breaks can play a role in the aging process and lead to the development of cancer. Now, with the help of infrared spectroscopy, scientists from the Departments of Biology and Chemistry at the University of Konstanz have successfully visualized the molecular processes that take place at the DNA strand breaks, in real-time.
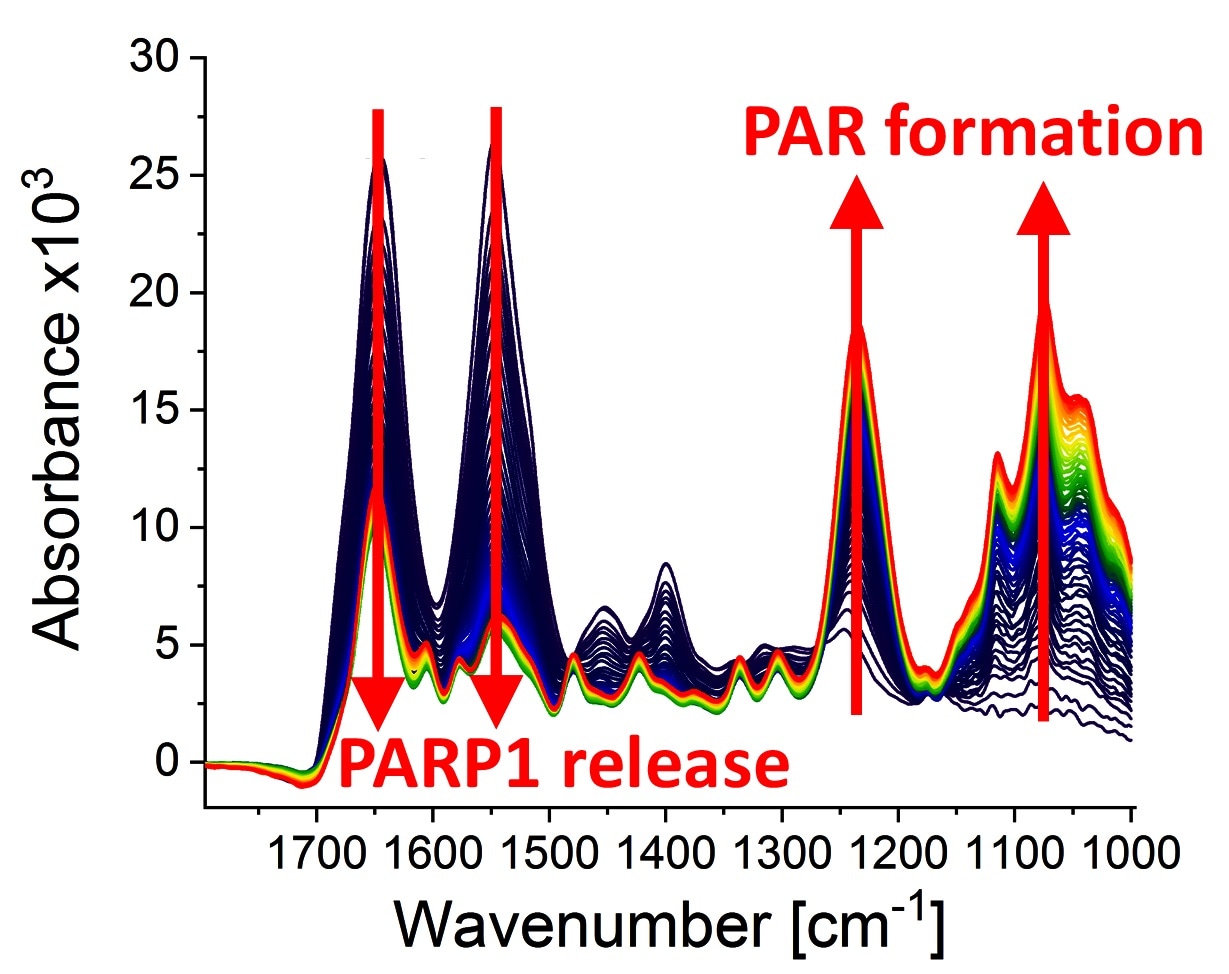
Infrared spectra at different points of time (0-79 minutes) after the poly(ADP-ribosyl)ation reaction started due to the addition of PARP1 substrate NAD+. The following can be observed: the dynamic formation of the biopolymer poly(ADP-ribose) (absorption bands at 1236 cm−1 and 1074 cm−1) and the detachment of PARP1 from the DNA strand break (absorption bands at 1645 cm−1 and 1548 cm−1). Image Credit: Modified from Krüger et al.
DNA damage usually and DNA strand breaks specifically take place daily in all the cells of the human body. This can be attributed to the internal effects like free radicals, which are generated during cellular respiration, inflammatory processes, and also external ones, like cosmic background radiation or X-rays during medical diagnostic measures.
DNA strand breaks can cause mutations or cell death and thus lead to the aging process or cancer development, in the long term.
DNA repair by PARP1
Cells contain molecular tools to repair these DNA strand breaks in a very efficient manner. The enzyme poly (ADP-ribose) polymerase 1 (PARP1) is one of the molecular tools that spot DNA strand breaks and thus triggers downstream repair processes.
When bound to a DNA strand break, PARP1 is (catalytically) stimulated and uses the substrate nicotinamide adenine dinucleotide (NAD+) to create a chain-shaped biopolymer called poly (ADP-ribose) (PAR). This acts as a signal transmitter in the cell and mediates the additional DNA damage response.
In the subsequent course of the process, PARP1 again detaches from the damage site, thereby clearing the way for further steps in the DNA repair process. This process holds medical significance, even more so as PARP1’s pharmacological inhibitors have been newly introduced into cancer treatments.
The researchers from the University of Konstanz (working groups of Professor Aswin Mangerich and Professor Alexander Bürkle, Department of Biology, and a working group of Professor Karin Hauser, Department of Chemistry) have successfully and comprehensively visualized the biochemical processes met by PARP1 at a DNA strand break.
For this purpose, the researchers employed a unique method of infrared spectroscopy (ATR-FTIR), which had been effectively applied in an earlier study on the interactions of the tumor suppressor protein called p53 with PAR and DNA.
Real-time observations using infrared spectroscopy
What is special about our new study is that we can now investigate the molecular processes that PARP1 undergoes at DNA strand breaks in real time. This enabled us to unveil dynamic changes in the protein structure and thus gain further insights into the underlying mechanisms.”
Dr Annika Krüger, Researcher, University of Konstanz
Dr. Krüger worked on the study as part of her, by now effectively concluded, doctoral thesis. While working on her doctoral thesis, Dr. Krüger was supported by the Research School Chemical Biology, the Zukunftskolleg of the University of Konstanz, and the Konstanz Collaborative Research Centre 969 “Chemical and Biological Principles of Cellular Proteostasis”. Dr. Krüger is presently pursuing research at the well-known Karolinska Institute in Stockholm, Sweden.
By principle, this spectroscopic technique can also be employed to study other enzymatic processes that occur at the DNA, comprehensively, and with molecular resolution. In the long term, this may lead to a deeper understanding of the mechanisms of aging and cancer development, and also the mode of action of anticancer drugs.
The research was published in the latest issue of the scientific journal Nature Communications.
Source:
Journal reference:
Krüger, A., et al. (2020) Real-time monitoring of PARP1-dependent PARylation by ATR-FTIR spectroscopy. Nature Communications. doi.org/10.1038/s41467-020-15858-w.