Researchers from Cold Spring Harbor Laboratory (CSHL) have discovered that the growth of pancreatic cancer cells can be stopped by disrupting the way cholesterol is stored by the cells.
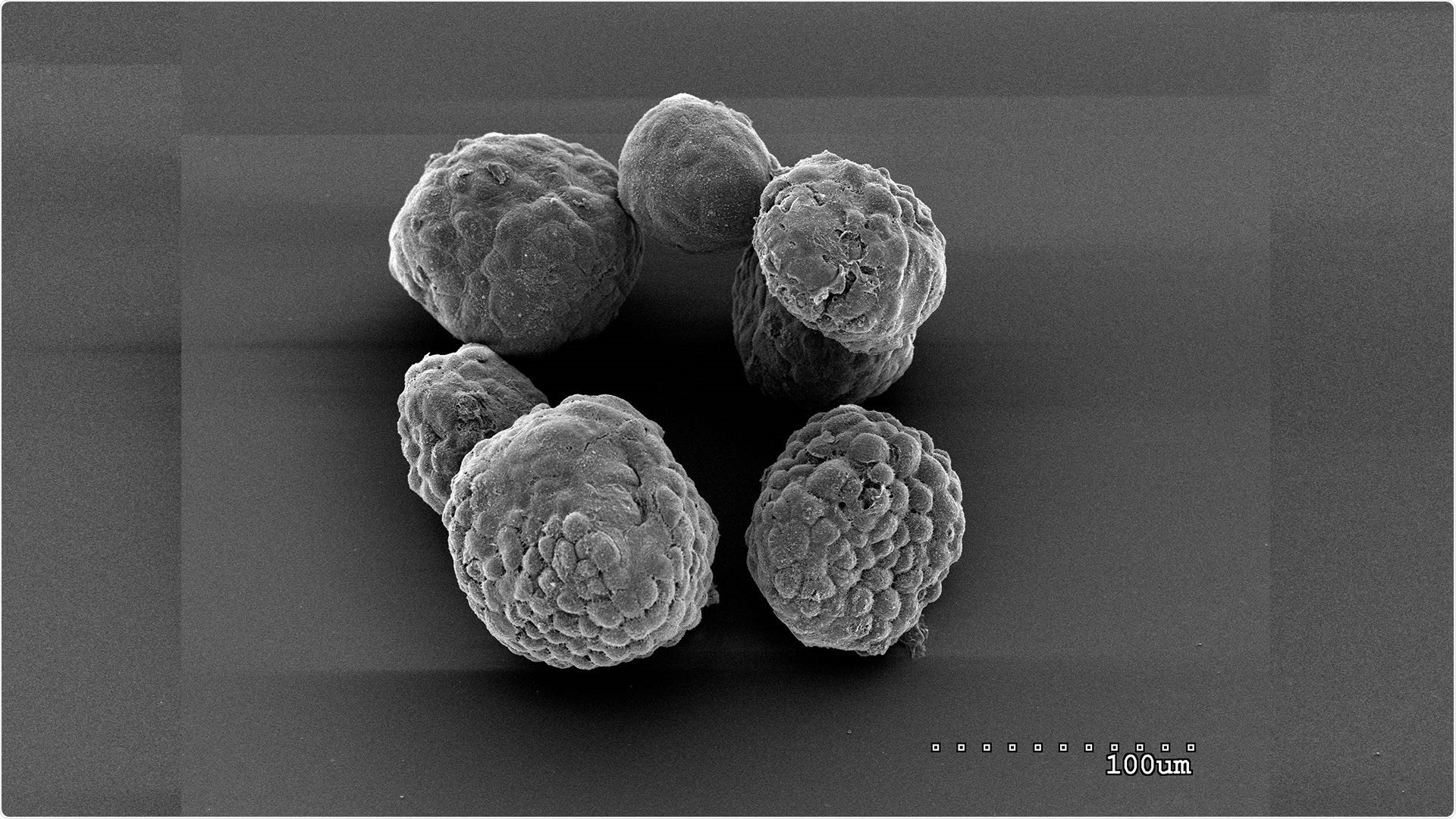
Organoids of mouse pancreatic tumor cells grown “ex vivo,” outside the body. Organoids are used as a model system to study tumor biology and treatments. Image Credit: Tobiloba Oni, Tuveson lab/Cold Spring Harbor Laboratory.
The team’s findings in both laboratory-grown pancreatic models and mice present a new method to fight this deadly disease.
Published in the Journal of Experimental Medicine, the study was headed by David Tuveson, a Professor at CSHL. Tuveson’s research team wished to find out why pancreatic cancer cells, similar to several cancer cells, produce high quantities of cholesterol.
Although cholesterol is a major component of cell membranes, the researchers established that pancreatic cancer cells make far more cholesterol than required by them to support their own growth.
This is unusual, because the cholesterol pathway is one of the most regulated pathways in metabolism.”
Tobiloba Oni, Graduate Student, Cold Spring Harbor Laboratory
Oni works in Tuveson’s laboratory.
He explained that a majority of the cells make only as much cholesterol as they require. Once these cells have a sufficient amount, they rapidly shut down the synthesis pathway.
However, Oni and his collaborators, including Giulia Biffi—a former postdoctoral fellow in Tuveson’s laboratory—discovered that most of the cholesterol made by the cancer cells are converted into a form of cholesterol by the cells themselves. This form of cholesterol can be preserved inside the cell. Free cholesterol never builds up, and the synthesis pathway continues to release more of this.
Cancer cells present in the pancreas appear to drive this hyperactive cholesterol synthesis. According to the researchers, this might be because these cells are exploiting other kinds of molecules produced by the same pathway.
Thanks to an enzyme known as sterol O-acyltransferase 1 (SOAT1), these cancer cells have the ability to keep the pathway running and sustain their supply. This enzyme changes free cholesterol to its stored form, which is abundantly present in the pancreatic cancer cells.
When the scientists removed the SOAT1 enzyme by genetic manipulation, inhibiting cells from changing and storing their cholesterol, cancer cells ceased to proliferate. In experiments conducted on animals, tumor growth was stopped by eliminating the enzyme.
Most significantly, the researchers observed that the elimination of the SOAT1 enzyme only affected the cells that harbored mutations in two copies of a tumor suppressor gene called p53. This genetic modification supports cancer growth and is very common in patient tumors.
Oni stated that normal pancreas cells worked equally well without the enzyme in the experiments conducted by the team, and that makes the SOAT1 enzyme a potential therapeutic target. He believes that scientists will be able to design a drug that particularly blocks the enzyme, damaging cancer cells but without affecting the normal cells.
Source:
Journal reference:
Oni, T., et al. (2020) SOAT1 promotes mevalonate pathway dependency in pancreatic cancer. Journal of Experimental Medicine. doi.org/10.1084/jem.20192389.