Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Image Credit: Christopher Wiley, PhD.
This breakthrough is predicted to have a crucial role in the measures to develop treatments that can fight against an extensive range of chronic age-related conditions varying from lung disease to arthritis to glaucoma and Alzheimer’s disease.
This biomarker is an exclusive signaling lipid metabolite, which is usually mainly intracellular, but is released when senescent cells are forced to die. This metabolite can be detected in urine and blood, which makes its non-invasive testing viable.
An increasing list of senolytic drugs is being developed, and thus, the detection of this metabolite through a companion test could confirm the performance of senolytic candidates.
The list of age-related diseases definitively linked to cellular senescence keeps growing, as does the number of biotech companies racing to develop drugs to eliminate senescent cells. While the field has never been more promising, the lack of a simple biomarker to measure and track efficacy of these treatments has been a hindrance to progress. We are excited to bring this new biomarker to the field and look forward to it being used in the clinic.”
Judith Campisi, PhD, Study Senior Scientist and Professor, Buck Institute
Understanding senescence and identifying lipids as a new and potent component of the SASP
When cellular senescence occurs, damaged or stressed cells stop dividing permanently as a putative mechanism to protect against cancer. Senescent cells actually do not die—they discharge a stew of bioactive molecules that boost wound healing and chronic inflammation. The latter has a major role in several age-related diseases since the cells tend to accumulate over time.
This bioactive stew is called the senescence-associated secretory phenotype, or SASP. Researchers have performed in-depth studies of its composition and deleterious effects.
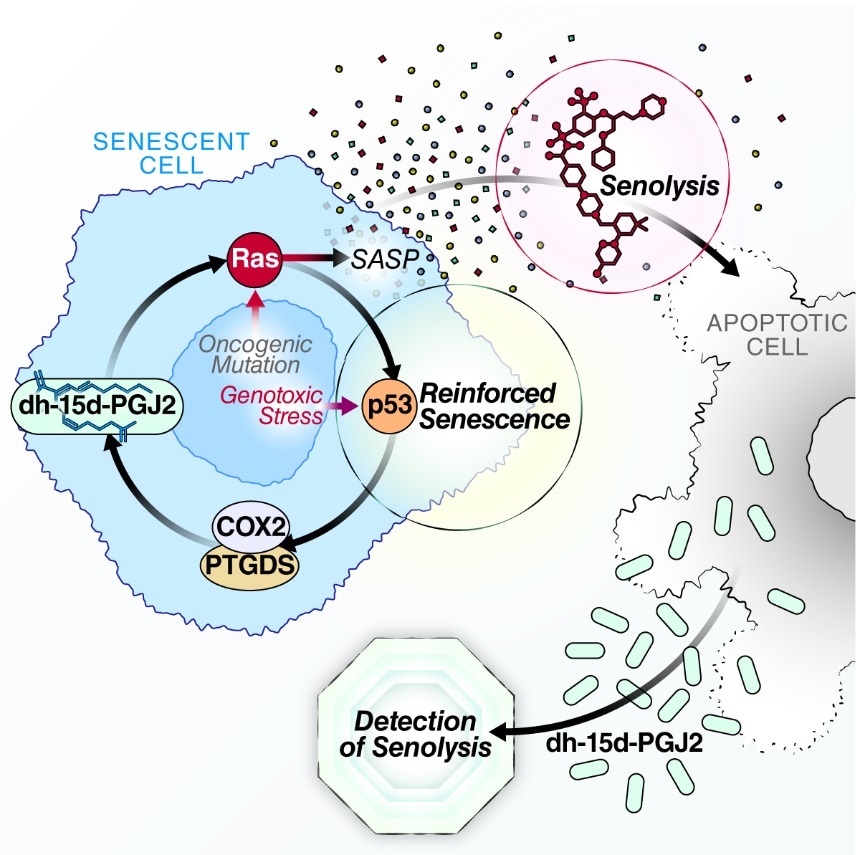
This study was conducted in human cell culture and mice, revealing that senescent cells also produce a wide range of oxylipins—bioactive metabolites produced by the oxygenation of polyunsaturated fatty acids.
Lipid components of the SASP have been vastly understudied. The biosynthesis of these signaling lipids promotes segments of the SASP and reinforces the permanent growth arrest of senescent cells. This work provides a new way of understanding and studying senescence-driven pathology.”
Christopher Wiley, PhD, Study Lead Scientist and Former Assistant Research Professor, Buck Institute
Wiley is now at the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston.
In several inflammatory conditions, such as cardiovascular disease and pain response, oxylipins are involved. A number of commonly used drugs, like ibuprofen and aspirin, act by inhibiting oxylipin synthesis.
The biomarker is unique to senescent cells and may be of interest in cancer research
According to Wiley, senescent cells alter their fatty acid metabolism and perform it such that free polyunsaturated fatty acids get accumulated within the arrested cells, where they are used to produce oxylipins.
One of these fatty acids—15-deoxy-delta-12, 14-prostaglandin J2 (dihomo-15d-PGJ2)—was identified by scientists as exclusive to senescent cells. It gets accumulated within senescent cells and is discharged when the cells die.
As part of this study, mice were administered chemotherapy, for inducing large-scale senescence, and then a senolytic drug. The biomarker could be detected only in the urine and blood of mice treated with both chemotherapy and the senolytic, but not with either one only. This confirmed the specificity for senolysis.
The team also demonstrated that dihomo-15d-PGJ2 had a functional role in senescence. Blocking its synthesis enabled a subset of cells to prevent senescence and continue their division. It also had a less inflammatory SASP profile. Adding dihomo-15d-PGJ2 to non-senescent cells induced them into senescence by stimulating RAS—a cancer-promoting gene known to cause senescence.
We hope that identifying and including these bioactive lipids as part of the SASP will encourage researchers working in a broad range of fields to take a new look at cellular senescence. The fact that one of these lipids ends up being a simple non-invasive biomarker for tracking the efficacy of treatments is a huge plus for those of us working to stem the ravages of age-related disease.”
Judith Campisi, PhD, Study Senior Scientist and Professor, Buck Institute
Source:
Journal reference:
Wiley, C. D., et al. (2021) Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metabolism. doi.org/10.1016/j.cmet.2021.03.008.