Increasing evidence points to the fact that gut microbiota performs a vital role in regulating the advancement of neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD); however, the molecular mechanism behind such microbe-host interaction is still not clear.
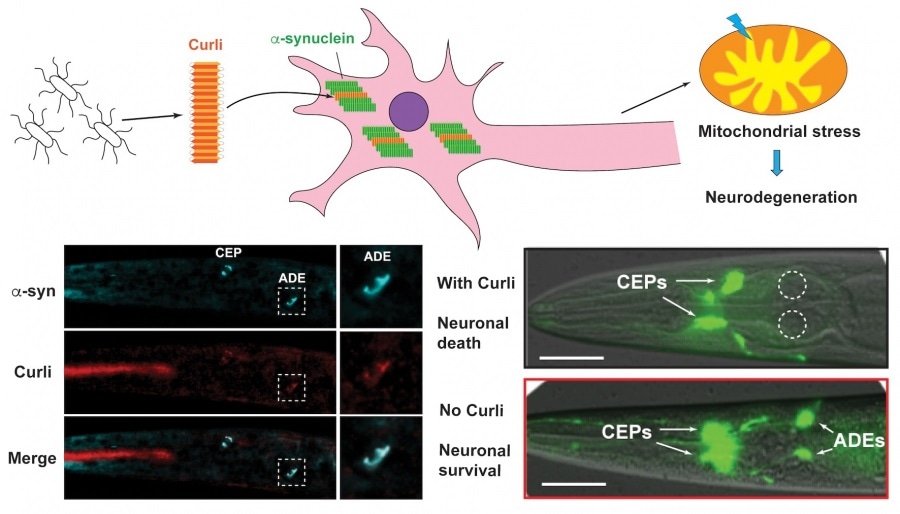
Bacterial curli promotes the aggregation of α-synuclein through cross-seeding, which leads to mitochondrial stress and neurodegeneration. Image Credit: The University of Hong Kong.
A team of scientists headed by Dr. Chaogu Zheng from the School of Biological Sciences at the University of Hong Kong (HKU) found that bacteria-derived curli amyloid fibril facilitates neurodegeneration in the host.
This novel research offers direct evidence to show that bacteria can produce proteins that form an amyloid fibril, which upon entering the host neurons facilitates protein aggregation and neurodegeneration.
Preventing the bacteria’s ability to secrete similar proteins may prove a preventative treatment for neurodegenerative diseases. The results of the study have been published in Proceedings of the National Academy of Sciences of the United States of America (PNAS), a leading multidisciplinary journal.
The secret weapon of gut microbiota
Neurodegenerative diseases occur due to protein aggregation in the neurons. Research works from the recent past indicate that gut bacteria might play a crucial role in the pathogenesis of neurodegenerative diseases.
For instance, an antibiotic treatment that destroys the majority of the bacteria in the gut can help improve the pathophysiology of PD in mice. In spite of the upcoming idea of the “microbiota-gut-brain” association, there is less knowledge about the bacterial molecules that regulate the advancement of neurodegeneration.
To tackle the issue, the scientists screened for bacterial E. coli genes whose deletion mitigates the symptoms of PD in an animal model of PD. This animal employed for the research is a microscopic nematode (worm) called Caenorhabditis elegans.
It is commonly used by researchers globally as a vital model organism for biological research. As this animal feeds on bacteria, the scientists developed it into a powerful tool to examine bacteria-host interaction.
From a genome-wide screen, Dr Zheng’s group pointed out 38 bacterial genes that can cause neurodegeneration in the animal host. Two of these genes code for proteins that form curli, a type of bacterial amyloid fiber.
The scientists later demonstrated that bacterial curli enters the neurons to cross-seed the human amyloid α-synuclein and facilitates its aggregation, which results in mitochondrial dysfunction, proteotoxicity, and neuronal death. Genetically removing curli genes or pharmacologically inhibiting curli formation considerably lowered the PD symptoms and enhanced neuronal functions.
A potential therapeutic approach
Moreover, scientists identified that curli also facilitated neurodegeneration in animal models of Amyotrophic lateral sclerosis, Alzheimer’s disease, and Huntington’s disease, indicating that the bacteria-secreted curli might have harmful effects in a range of neurodegenerative disorders. Targeting curli secretion in the gut might represent a general therapeutic approach to inhibit or slow down the advancement of protein aggregation diseases.
One of the interesting findings in this study is that a polyphenol called EGCG from green tea extracts can almost completely inhibit curli secretion in bacteria and has amazing effects in suppressing neurodegeneration. This is consistent with the observation that drinking green tea has beneficial effects in preventing neurodegenerative diseases.”
Dr Chenyin Wang, Study First Author, University of Hong Kong
This study unravels a new perspective to develop preventative measures for neurodegenerative diseases by focusing on bacterial curli secretion in the human gut.
This study established a new paradigm for understanding how bacterial components from the gut microbiome can influence neuronal health in animals.”
Dr Chaogu Zheng, School of Biological Sciences, University of Hong Kong
Dr. Zheng indicated the significance of the research by the above statement. Dr. Zheng intends to further proceed with the research on other bacterial molecules determined from the screen and examine how they affect host neurodegeneration. Dr. Zheng anticipates that a thorough knowledge of the bacteria-host interaction in the context of neurodegeneration can help find out novel therapeutic targets for neurodegenerative disorders.
Source:
Journal reference:
Wang, C., et al. (2021) Genome-wide screen identifies curli amyloid fibril as a bacterial component promoting host neurodegeneration. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2106504118.