A geographic and genetic analysis of variants of SARS-CoV-2—the virus causing COVID-19—and related viruses in animals and humans might offer proof of interspecies transmission globally.
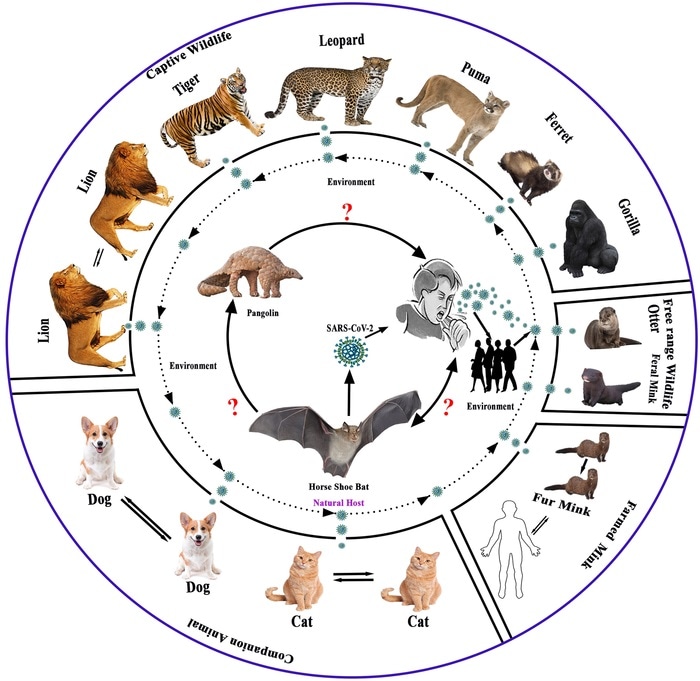
Possible evolution and transmission pathways of SARS-CoV-2 and its related virus at animal-human- ecosystem interface. Image Credit: Islam, et al., 2021, PLOS ONE, CC-BY 4.0.
Dr Ariful Islam from the EcoHealth Alliance and the Bangladesh Institute of Epidemiology, Disease Control and Research, and coworkers carried out research based on these. Their findings were published on December 15th, 2021, in PLOS ONE, an open-access journal.
Earlier studies have indicated that SARS-CoV-2 would have originated through genetic changes that took place among closely related viruses in horseshoe bats. There are also reports suggesting that the virus can spread from humans to wild and domesticated animals (called spillback).
Still much more information needs to be comprehended on the evolutionary dynamics, epidemiology, and genetic relationships between SARS-CoV-2 and related viruses in animals worldwide.
Dr Islam and coworkers carried out an extensive analysis of the genome sequences of SARS-CoV-2-related viruses seen in pangolins and bats. They also analyzed the SARS-CoV-2 strains seen in animals globally, including cats, dogs, and lions.
Genetic analysis revealed that SARS-CoV-2 strains seen in animals are closely related to strains seen in humans in the corresponding geographic regions—in addition to France, Germany, Denmark, and Spain—which proves the theory that human to animal transmission has taken place globally. In the future, in case the virus establishes in animal hosts, that animal species could act as a SARS-CoV-2 reservoir.
The scientists also quantified the amount to which genetic mutations linked with major SARS-CoV-2 subtypes and variants are seen in animal species in diverse regions. The emerging variants of concern—like Alpha, Delta, and Mu-variants—were identified in various countries in species like gorillas, lions, dogs, and cats.
These variants also revealed prominent genetic similarities with human SARS-CoV-2 sequences. For instance, American mink and cats were often infected with a subtype of the virus called the GR clade (31.6% and 49.7% of samples respectively), which is also mostly found in humans, confirming the likelihood of interspecies transmission.
But, the majority of the dogs were affected by various subtypes, clade O (66.7%). The researchers suggest that this demonstrates a specific affinity of clade O for dogs. The SARS-CoV-2 Alpha variant had just 4.8% of dog samples and 2.6% of cat, but 77.3% of lion and 66.7% of gorilla samples.
The genetic analysis carried out demonstrated a very high similarity between SARS-CoV-2 and related viruses seen in multiple horseshoe bat species and also a high similarity with related viruses in the Malayan pangolin. The analysis favors the hypothesis that SARS-CoV-2 originated from closely related bat viruses that genetically recombined with each other, and also that the virus passed through pangolins.
Animals seem to be susceptible to SARS-CoV-2 and might contribute to its spread. The scientists based on their observations request continued genetic monitoring of SARS-CoV-2 in animals.
They also necessitate the prevention of contact between animals and infected humans along with vaccination of pets, farm animals, and zoo animals. The scientists also advise surveillance at the human-animal interface to identify and prevent the advent of future viruses.
“Spillover and spillback of SARS-CoV-2 have been proved, and the mutant strain of the virus is still dominating. It is our responsibility not to destroy the natural habitats of wildlife. As bats play an important role in maintaining a healthy ecosystem, we need to work on how to live safely with bats. We should not wait until the next pandemic, and we need to apply the One Health approach to secure a healthier future for the world,” the authors added.
Source:
Journal reference:
Islam, A., et al. (2021) Spatial epidemiology and genetic diversity of SARS-CoV-2 and related coronaviruses in domestic and wild animals. PLoS ONE. doi.org/10.1371/journal.pone.0260635.