Raphidocystis contractilis belongs to Heliozoa, a group of eukaryotes that is generally seen in brackish, fresh, and seawater. The organisms belonging to this group have finger-like arms, known as axopodia, which extend out from their body, giving them a sun-like appearance. So, they are also called “solar worms”.
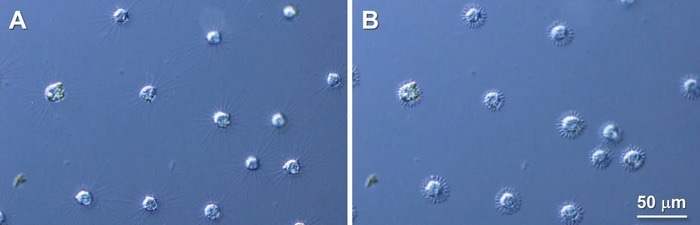 Rapid axopodial shortening seen in Raphidocystis contractilis, before (A) and after (B) mechanical stimulation. Image Credit: Okayama University.
Rapid axopodial shortening seen in Raphidocystis contractilis, before (A) and after (B) mechanical stimulation. Image Credit: Okayama University.
Each axopodium is made up of the proteins, alpha-beta tubulin heterodimers, which form filaments known as microtubules. In reaction to external stimuli, R. contractilis may remove its axopodia extremely fast. The mechanism causing this rapid arm shortening, however, remains unknown.
A group of Okayama University researchers led by Professor Motonori Ando, Dr Risa Ikeda (both from the Laboratory of Cell Physiology), and Associate Professor Mayuko Hamada (from the Ushimado Marine Institute), of Okayama University, Japan, investigated the mechanism behind one of the fastest cell movements in the living world.
Recently, a wide variety of heliozoans have been discovered in various hydrospheres in the Okayama Prefecture, making it clear that several species of sun worms inhabit the same environment. We are trying to unravel the mysteries around these protozoans and gradually expand the horizons of our knowledge.”
Motonori Ando, Professor, Laboratory of Cell Physiology, Okayama University
The researchers began their research by immunolabelling the tubulin protein and monitoring its movement before and after axopodial contraction. Tubulins were discovered to be distributed systematically all along the length of the axopodia before shortening, however after axopodial withdrawal, they quickly accumulated near the cell surface.
This prompted them to conclude that during the quick axopodial withdrawal, the microtubules instantly broke into tubulin. Microtubule degradation, on the other hand, is not a fast process; it takes a bit of time.
So, how could R. contractilis execute this change so fast?
The researchers speculated that this was possible if microtubules split at many sites at the same time. The authors set out to uncover the proteins and genes involved in the instantaneous cleavage of microtubules in R. contractilis to validate their theory. On November 21st, 2022, their results were published online in The Journal of Eukaryotic Microbiology.
The researchers found nearly 32,000 genes in R. contractilis using de novo transcriptome sequencing (an analysis of the genes expressed during a specific time in a cell). This gene set was most akin to protozoans (single-celled organisms), followed by metazoans (multicellular organisms with well-differentiated cells; this includes humans and other animals).
The obtained gene set’s homology and phylogenetic analysis showed multiple genes (and their associated proteins) involved in microtubule disruption. Among these, katanin p60, kinesin, and calcium signaling proteins were the most important.
Katanin p60 was found to be involved in the regulation of axopodial arm length. Several kinesin gene duplicates were discovered. Kinesin-13, a significant microtubule destabilizing protein, was discovered to play a crucial role in the fast contraction of axopodia among the recognized kinesins.
Calcium signaling genes control the entry of calcium ions into the cell and the triggering of axopodial withdrawal.
The researchers also discovered a lack of genes associated with flagellar formation and motility, revealing that R. contractilis axopodia did not evolve from flagella. Even though many genes remain unclassified, the newly identified gene set will serve as a reference for future research aimed at understanding R. contractilis axopodial motility.
Heliozoan axopodia have the ability to operate as a sensitive sensor. They are capable of detecting minute changes in their surroundings, such as the presence of heavy metal ions and anticancer drugs.
We believe that the axopodial response of heliozoa can be used as an index to develop temporary detection and monitoring devices for environmental and tap water pollution. It can also be used as a novel bioassay system for the primary screening of novel anticancer drugs. In the future, we plan to continue to work together as a team to enhance basic and applied research on these organisms.”
Motonori Ando, Professor, Laboratory of Cell Physiology, Okayama University
Heliozoans have once again shown that a single cell has the ability to influence the world.
Source:
Journal reference:
Ikeda, R., et al. (2022) De novo transcriptome analysis of the centrohelid Raphidocystis contractilis to identify genes involved in microtubule-based motility. The Journal of Eukaryotic Microbiology. doi.org/10.1111/jeu.12955.