Dr Catherine Mummery, a consultant neurologist at UCL Queen Square Institute of Neurology & the National Hospital for Neurology and Neurosurgery, has led a trial. The trial signifies for the first time that a “gene silencing” method has been taken in Alzheimer’s and dementia disease.
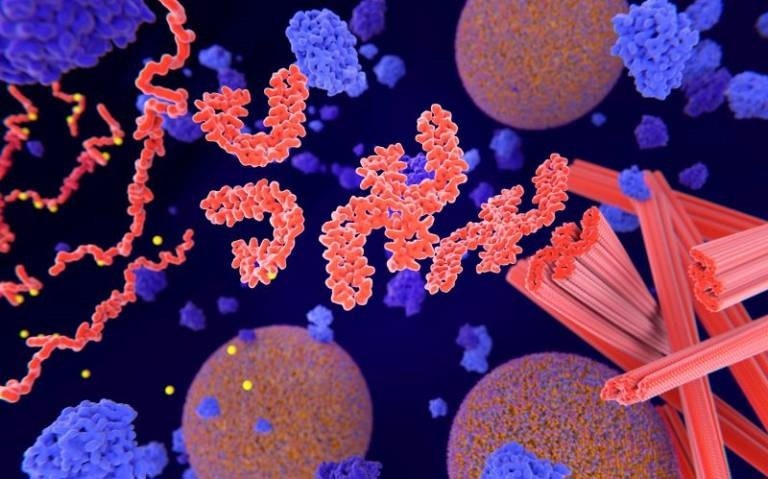
Pathological phosphorylation (yellow) of Tau proteins (red-orange) leads to disintegration of microtubuli in the neuron axon an aggregation of the tau proteins. The transport of synaptic vesicles (orange-blue) is interrupted. Image Credit: Selvanegra on iStock.
The method employs a drug known as BIIB080 (/IONIS-MAPTRx), an antisense oligonucleaotide (utilized to curb RNA that produces a protein), to “silence” the gene coding for the tau protein—called the microtubule-associated protein tau (MAPT) gene. This avoids the translation of the gene into the protein in a reversible and doseable way. In addition, it will minimize the generation of that protein and change the disease course.
More trials will be required in bigger groups of patients to identify whether this results in clinical benefit, but the phase 1 outcomes that were published in Nature Medicine—with findings from 46 patients—are the initial signal that this approach has a biological effect.
Currently, there are no treatments that target tau. The drugs aducanumab and lecanemab—approved recently for application in certain situations through the FDA—aim for a separate disease mechanism in AD, which is the accumulation of amyloid plaques*.
The phase 1 trial focused on the BIIB080’s safety, what it does in the body, and how well it aims the MAPT gene. It involved the UCL Dementia Research Centre, was financially aided by the NIHR UCLH Biomedical Research Centre, was supported by the NIHR UCLH Biomedical Research Centre, and happened at the Leonard Wolfson Experimental Neurology Centre at NHNN.
A total of 46 patients, with a mean age of 66, enrolled in the trial. It happened between 2017 and 2020. Three drug doses, offered through intrathecal injection (an injection into the nervous system through the spinal canal), were looked at by the trial, and they were compared with the placebo.
Outcomes reveal that the drug was well tolerated, with every patient finishing the treatment period and more than 90% finishing the post-treatment period.
Mild or moderate side effects, where the most common being a headache following drug injection, were experienced by patients in both the placebo and treatment groups. Nevertheless, no extreme adverse events were observed in patients who were given the drug.
The study team also looked at two kinds of tau protein in the central nervous system (CNS), a dependable sign of disease—over the study period.
They discovered a greater than 50% reduction in total tau and phosphor tau concentration levels in the CNS following 24 weeks in the two treatment groups that got the highest drug dosage.
We will need further research to understand the extent to which the drug can slow progression of physical symptoms of disease and evaluate the drug in older and larger groups of people and in more diverse populations. But the results are a significant step forward in demonstrating that we can successfully target tau with a gene silencing drug to slow—or possibly even reverse—Alzheimer’s disease, and other diseases caused by tau accumulation in the future.”
Dr Catherine Mummery, Consultant Neurologist, University College London
Source:
Journal reference:
Mummery, C., et al. (2023) Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: a phase 1b, randomized, placebo-controlled trial. Nature Medicine. https://doi.org/10.1038/s41591-023-02326-3.