Tokyo Metropolitan University researchers have uncovered crucial aspects of the DNA repair mechanism within human bodies.
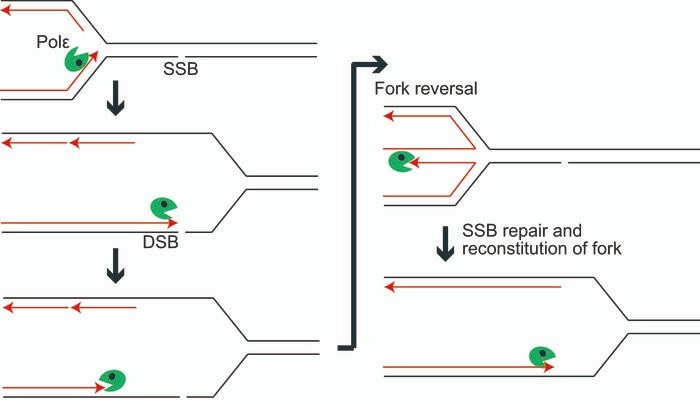 When an SSB appears, polymerase epsilon approaches the nick before retracing its steps thanks to its exonuclease, allowing fork reversal to take place. The nick is repaired as an SSB, and replication can continue as usual. Image Credit: Tokyo Metropolitan University
When an SSB appears, polymerase epsilon approaches the nick before retracing its steps thanks to its exonuclease, allowing fork reversal to take place. The nick is repaired as an SSB, and replication can continue as usual. Image Credit: Tokyo Metropolitan University
In a groundbreaking revelation, a research team from the university demonstrated that the “proofreading” segment of the DNA-replicating enzyme polymerase epsilon plays a vital role in ensuring the safe termination of replication at damaged sections of the DNA strand.
This discovery ultimately safeguards DNA from severe damage. The newfound knowledge provides scientists with insights to enhance the effectiveness of anti-cancer drugs and develop novel diagnostic methods.
Human DNA faces constant threats, with approximately 55,000 single-strand breaks (SSBs) occurring daily in the strands composing DNA helices within individual cells.
When polymerases, molecules responsible for replicating DNA strands, attempt to create new helices from strands with breaks, they risk breaking the helix, resulting in what is known as a single-ended double-stranded break (seDSB).
Fortunately, cells possess innate mechanisms to address strand damage, including homology-directed repair (HDR) for fixing double-stranded breaks and “fork reversal,” which involves reversing the replication process to prevent single-strand nicks from evolving into DSBs in the first place.
While the precise mechanism of fork reversal has remained elusive, understanding how DNA damage is averted is critical not only for cancer prevention but also to ensure the efficacy of cancer drugs relying on inducing DNA damage.
An international team, spearheaded by Professor Kouji Hirata from Tokyo Metropolitan University, has illuminated the workings of fork reversal. Their focus was on polymerase epsilon, an enzyme responsible for synthesizing new DNA from an unzipped portion of the DNA.
The researchers revealed that the exonuclease, the "proofreading" component of the polymerase ensuring copy accuracy, plays a pivotal role, offering a rare insight into the largely unknown molecular mechanism governing fork reversal.
Notably, cells deficient in the exonuclease segment exhibited heightened susceptibility to exposure to the anti-cancer drug camptothecin (CPT). Suppressing PARP, another factor known to impact fork reversal, also resulted in increased cell death.
Interestingly, simultaneous suppression of both did not lead to further increases in cell death beyond what was observed with PARP alone, suggesting a collaborative action between PARP and polymerase epsilon exonuclease in triggering fork reversal.
Additionally, the team investigated cells with disrupted BRCA1 gene coding (associated with breast cancer susceptibility).
The additional deficiency of the exonuclease significantly increased sensitivity to CPT, surpassing expectations from either defect alone. Given the link between BRCA1 deficiency and a heightened risk of breast cancer, targeting the exonuclease could enhance the effectiveness of drug treatments.
The significance of this research is manifold. It not only demonstrates that drugs targeting the polymerase epsilon exonuclease can augment the effects of anti-cancer drugs but also highlights the presence of exonuclease defects in various cancers, including intestinal cancer. This suggests impaired fork reversal capability in such cells, presenting a promising target for future diagnostics and treatments.
Source:
Journal reference:
Ahmad, T., et al. (2023) The proofreading exonuclease of leading-strand DNA polymerase epsilon prevents replication fork collapse at broken template strands. Nucleic Acids Research. doi.org/10.1093/nar/gkad999.