Alzheimer's disease (AD) continues to be one of the most difficult and common neurodegenerative diseases, impacting millions of people globally. Researchers from the lab of Wim Annaert (VIB-KU Leuven) have discovered a novel mechanism that may be related to the initial phases of AD, according to a recent study published in Developmental Cell.
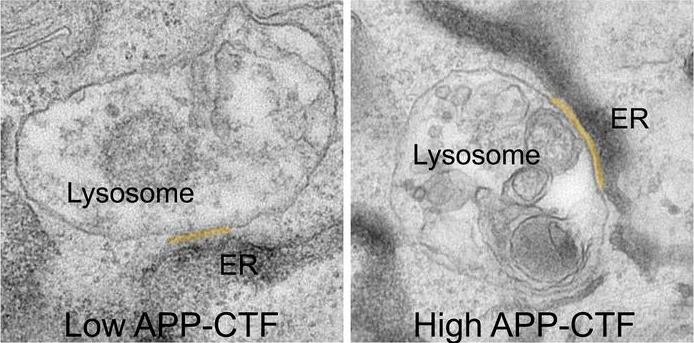 APP-C-Terminal Fragments (APP-CTFs) accumulate between the endoplasmic reticulum and the lysosomes. Image Credit: Vlaams Instituut Voor Biotechnologie.
APP-C-Terminal Fragments (APP-CTFs) accumulate between the endoplasmic reticulum and the lysosomes. Image Credit: Vlaams Instituut Voor Biotechnologie.
They showed how communication between cellular compartments essential for waste disposal and calcium storage is disrupted by a fragment of the amyloid precursor protein (APP), known as APP-CTF.
This may be an early event leading up to neuronal cell death. These results imply that preventing APP-CTF accumulation must be taken into consideration to develop more potent treatments, which may have implications for the development of new AD treatments.
Memory loss, behavioral abnormalities, and a progressive loss of cognitive function are the hallmarks of Alzheimer's disease. Amyloid plaques, or aggregates of β-amyloid (Aβ) peptides, are one of the obvious signs of Alzheimer's disease and are the breakdown products of amyloid precursor protein (APP). Even before a decline in cognitive function is noticed, these Aβ-fragments start to accumulate in neurons early in the disease.
According to recent research, the APP protein may be involved in these early phases of AD brain development, possibly even occurring before plaque formation. Until recently, the mechanism underlying this was unknown.
The Wim Annaert lab at the VIB-KU Leuven Center for Brain & Disease Research has discovered a mechanism that explains how APP may be involved in these early stages of AD, according to their most recent research. This finding may point research on AD in a new direction and change how it is treated.
Disrupting Cellular Communication
The cell membranes of brain cells contain APP. The brain breaks down and eliminates old APP molecules while continuously producing new ones. The final enzymatic scissors in this process, gamma-secretase, produce the well-recognized and extensively researched Aβ peptides in AD.
It was long believed that the best way to stop the synthesis of harmful Aβ fragments would be to block gamma-secretase. On the other hand, this causes their precursors, known as APP-C-Terminal Fragments, or APP-CTFs, to accumulate.
It has been found by researchers that these fragments are toxic to neurons as well. They seem to gather between the lysosomes, or “waste bins” of neurons, which are essential for breaking down waste products in the cell, and the endoplasmic reticulum (ER), the area that is vital for lipid synthesis and calcium storage.
By doing so, APP-CTFs disrupt the delicate balance of calcium within lysosomes. This disruption triggers a cascade of events. The ER can no longer effectively refill lysosomes with calcium, leading to a buildup of cholesterol and a decline in their ability to break down cellular waste. This results in the collapse of the entire endolysosomal system, a crucial pathway for maintaining healthy neurons.”
Dr. Marine Bretou, Study First Author, Vlaams Instituut Voor Biotechnologie
The new study provides more evidence that endolysosomal dysfunction, which is seen in the very early stages of AD, may be caused by the APP-CTFs that arise from suppressing gamma-secretase.
A Paradigm Shift in Understanding the Early Stages of AD Pathogenesis
The understanding of the probable causes of the disease in the early stages of AD is greatly advanced by this research. This study's unexpected finding is that rather than Aβ, another piece of the same APP molecule may be to blame for these early stages. Given that current therapeutic approaches tend to overlook the toxic effects of other fragments, this has substantial implications for the goal of clearing amyloid plaques from the AD brain.
Other approaches concentrate on neuroinflammation or tau proteins, which are additional markers of AD progression that target subsequent events. To stop or even prevent AD, early intervention is probably essential.
The failure of clinical trials using gamma-secretase inhibitors may be explained by the fact that we were focusing on only one culprit and at a too late stage in the disease. Our research findings suggest that gamma-secretase modulators, which can help promote clearance of toxic APP-CTFs without blocking the enzyme completely, may be a more relevant target for early intervention in AD. The key might be finding the right balance between APP-CTF clearance and plaque prevention.”
Wim Annaert, Professor and Study Lead Author, Vlaams Instituut Voor Biotechnologie
In the future, the researchers will carry out more research on cellular homeostasis in the early phases of AD and will collaborate with colleagues to develop these modulators based on these new insights.
Source:
Journal reference:
Bretou, M., et al. (2024) Accumulation of APP C-terminal fragments causes endolysosomal dysfunction through the dysregulation of late endosome to lysosome-endoplasmic reticulum contact sites. Developmental Cell. doi.org/10.1016/j.devcel.2024.03.030