Reviewed by Lexie CornerMay 15 2025
Trillions of bacteria inhabit the human gut, playing roles beyond digestion, including influencing the immune system. These microbes interact with the body by releasing various chemicals that regulate the growth and activity of immune cells.
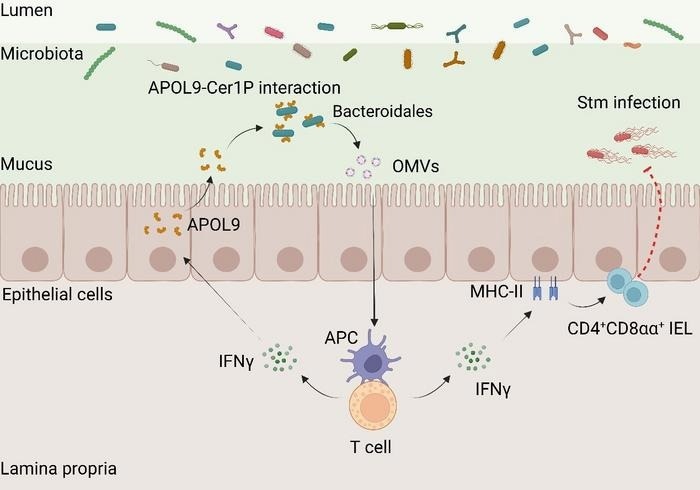 The proposed model for the novel apolipoprotein APOL9 that enhances mucosal immunity by activating the IFN-γ-MHC-II pathway through the induction of OMVs release in Bacteroidales. Image Credit: Professor Youcun Qian’s group
The proposed model for the novel apolipoprotein APOL9 that enhances mucosal immunity by activating the IFN-γ-MHC-II pathway through the induction of OMVs release in Bacteroidales. Image Credit: Professor Youcun Qian’s group
To maintain a healthy balance between host defense and microbial coexistence, the body relies on several defense mechanisms - including mucus, antimicrobial proteins, antibodies, and complement proteins - to regulate microbial activity and prevent harmful invasions. But one question has remained: can the human body specifically recognize and regulate certain bacteria within this vast microbial community?
In a study published in Nature on May 14, 2025, researchers addressed this question and identified an unexpected way in which the body communicates with gut bacteria to support intestinal health.
The study was led by Prof. Youcun Qian from the Shanghai Institute of Nutrition and Health (SINH), Chinese Academy of Sciences (CAS), and Prof. Xinyang Song from CAS’s Center for Excellence in Molecular Cell Science.
The team began by comparing gut lining samples from germ-free and conventionally raised mice using advanced proteomic techniques. This analysis led to the discovery of a previously unidentified protein, APOL9, which was significantly more abundant in mice with a normal gut microbiome. Further investigation showed that APOL9 is primarily produced by cells lining the intestine.
To explore which bacteria APOL9 interacts with, the researchers developed a technique called “APOL9-seq,” which combines flow cytometry and genomic sequencing. They found that APOL9 - and its human counterpart, APOL2 - binds selectively and strongly to a group of gut bacteria called Bacteroidales.
This specificity is mediated by a unique lipid molecule on the bacterial surface known as ceramide-1-phosphate (Cer1P). When researchers used gene editing to remove Cer1P from the bacteria, APOL9 could no longer bind to them. This is the first study to demonstrate that the host can detect and respond to specific bacterial lipid signatures.
Interestingly, APOL9 does not kill the bacteria it binds to, unlike typical antimicrobial proteins. Instead, it induces the bacteria to release outer membrane vesicles (OMVs) - tiny, nanometer-sized sacs filled with microbial compounds.
These OMVs are absorbed by the host's immune system and help boost immune readiness. The team found that OMVs enhance interferon-gamma (IFN-γ) signaling and upregulate MHC-II molecules on intestinal cells. These factors help train a specific subset of T cells (CD4+CD8αα+), which are crucial for maintaining immune balance in the gut.
To better understand APOL9’s role, the researchers used a mouse model in which the gene was deleted. When exposed to Salmonella, mice lacking APOL9 showed a weakened immune response and more severe infection. However, when these mice were treated with OMVs from bacteria, their immune activity increased and signs of illness were reduced.
The specific interaction between APOL9 and Cer1P highlights a finely tuned molecular 'dialogue' forged through long-term coevolution between the host and its microbiota. In the future, we plan to explore the role of human APOL2 and investigate whether modulating this pathway can strengthen the intestinal immune barrier.
Youcun Qian, Study Leading Researcher and Professor, Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences
This is the first study to show that a host protein can selectively recognize bacterial lipids and trigger beneficial immune responses. It also uncovers a novel mechanism by which the body actively shapes the gut microbiome, not just by tolerating microbes, but by communicating with them to maintain balance.
The findings suggest promising opportunities for developing next-generation therapies that modulate interactions between the microbiota and the immune system.
Source:
Journal reference:
Yang, T., et al. (2025) Targeting symbionts by apolipoprotein L proteins modulates gut immunity. Nature. doi.org/10.1038/s41586-025-08990-4.