Reviewed by Lexie CornerMay 20 2025
If DNA bridges remain between chromosomes after cell division, chromosome segregation can be disrupted, leading to genetic instability and increasing the risk of cancer. Researchers at UNIST and the Institute for Basic Science (IBS) have identified how a key protein acts during the final stages of cell division to remove these DNA bridges, often by severing them.
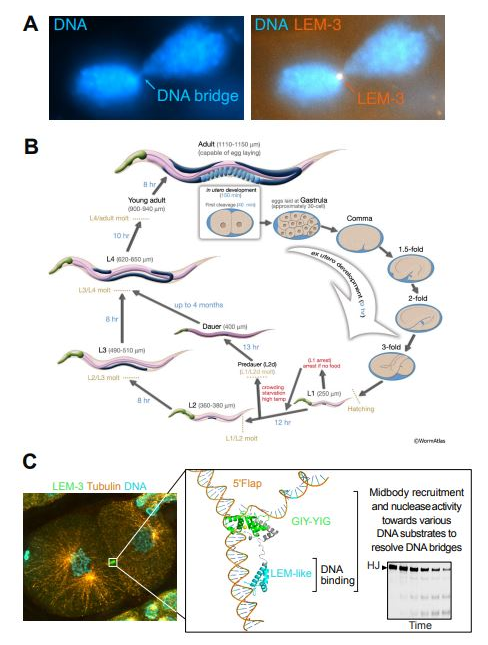 Functional analysis of the LEM-3 protein and the roles of its domains using the C. elegans model. (A) Evidence shows that LEM-3 accumulates at DNA bridges remaining between daughter cells during the final stages of cell division and contributes to their resolution. This was observed through fluorescence microscopy. (B) LEM-3 function was analyzed across various developmental stages using the C. elegans model, elucidating the mechanism by which DNA bridges are severed during cell division. (C) LEM-3 localizes to the midbody to recognize and cleave DNA bridges. Structural prediction and cleavage assays revealed that the LEM-like domain is responsible for DNA recognition, while the GIY-YIG domain directly mediates DNA cleavage. Image Credit: Institute for Basic Science
Functional analysis of the LEM-3 protein and the roles of its domains using the C. elegans model. (A) Evidence shows that LEM-3 accumulates at DNA bridges remaining between daughter cells during the final stages of cell division and contributes to their resolution. This was observed through fluorescence microscopy. (B) LEM-3 function was analyzed across various developmental stages using the C. elegans model, elucidating the mechanism by which DNA bridges are severed during cell division. (C) LEM-3 localizes to the midbody to recognize and cleave DNA bridges. Structural prediction and cleavage assays revealed that the LEM-like domain is responsible for DNA recognition, while the GIY-YIG domain directly mediates DNA cleavage. Image Credit: Institute for Basic Science
The molecular mechanism by which LEM-3 cuts DNA bridges during cytokinesis has been clarified, according to IBS Research Fellow Stephane Rolland and Professor Anton Gartner, Distinguished Professor at the UNIST Graduate School of Medicine and Associate Faculty Member of the IBS Center for Genomic Integrity.
Cell division is essential for replacing old cells and renewing tissues. In the human body, billions of cells divide daily: intestinal cells are replaced every 1–3 days, and skin cells every 2–3 weeks.
During this process, DNA must be accurately replicated and evenly distributed between daughter cells. However, incomplete DNA replication or chromosome entanglement can result in DNA bridges connecting the two cells. If unresolved, these bridges can lead to chromosomal instability, loss of genetic material, and an increased risk of cancer.
Previous research has shown that LEM-3 plays a critical role in resolving persistent DNA bridges, particularly when other repair mechanisms fail. LEM-3 localizes to the midbody, a narrow structure that connects two daughter cells in the final stages of division. When other DNA repair proteins were absent, most DNA bridges were removed, but without LEM-3, bridges remained, and cell division was impaired.
LEM-3 is a nuclease that cleaves DNA. In this study, researchers investigated how LEM-3 recognizes and cuts its substrates, and how its various domains contribute to its localization, catalytic function, and DNA binding. Although LEM-3 serves an essential role, the study also found that it can be harmful if mislocalized.
LEM-3 must be tightly regulated and excluded from the nucleus. The researchers identified a mutant form that mislocalizes to the nucleus, causing unintended DNA cleavage and embryonic lethality, highlighting the importance of controlling LEM-3 activity.
The study used the model organism Caenorhabditis elegans, whose LEM-3 protein is evolutionarily conserved in humans as ANKLE1.
Given that ANKLE1 has been linked to the development of breast and colorectal cancers, our findings may contribute to the development of new strategies for cancer prevention and therapy.
Anton Gartner, Distinguished Professor, Ulsan National Institute of Science & Technology
Source:
Journal reference:
Song, J., et al. (2025) Functional dissection of the conserved C. elegans LEM-3/ANKLE1 nuclease reveals a crucial requirement for the LEM-like and GIY-YIG domains for DNA bridge processing. Nucleic Acids Research. doi.org/10.1093/nar/gkaf265.