Reviewed by Lexie CornerJun 16 2025
Researchers at VIB and Ghent University have identified small antibodies that offer protection against several coronaviruses, including SARS-CoV-1 and multiple SARS-CoV-2 variants. These antibodies target a key region near the base of the virus’s spike protein, blocking its entry into cells.
The findings suggest that these antibodies may help counter both current and emerging variants.
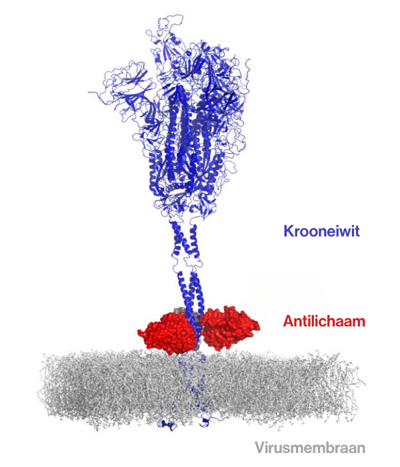 How the antibody attaches to the base of the crown protein. Image Credit: VIB
How the antibody attaches to the base of the crown protein. Image Credit: VIB
SARS-CoV-2, the virus that causes COVID-19, continues to evolve and cause illness. As a result, the search for effective treatments remains ongoing. Many antibody therapies become less effective as new variants emerge, especially when they target parts of the virus that frequently mutate.
To address this, a research team from the VIB-UGent Center for Medical Biotechnology, led by Prof. Xavier Saelens and Dr. Bert Schepens, took a different approach. They focused on a more stable region of the virus’s spike protein.
This region plays a key role in the fusion between the virus and human cells, marking the start of infection. Because this area changes little across coronaviruses and variants, it is a promising target for broader protection.
A Molecular Lock
The researchers used llamas - specifically one named Winter - to develop antibodies that target this part of the virus. Llamas produce a unique type of antibody that is smaller than those found in humans and most other mammals. Due to their size, these antibodies can access less-exposed regions of viral proteins.
The team identified several llama-derived antibodies that can neutralize different coronaviruses. These antibodies work by forming a molecular clamp. They bind to the S2 subunit of the spike protein and lock it in its original shape. As a result, the protein cannot expand into the active form needed to enter human cells.
Better Treatments
Animal studies have shown that these small antibodies provide strong protection against infection, even at low doses. The researchers also examined how quickly the virus might develop resistance. In the few cases where mutations allowed the virus to evade the antibodies, the resulting virus appeared to be much less infectious.
According to Bert Schepens, this feature makes the finding especially promising.
The region targeted by the antibodies is so crucial to the virus that it can hardly mutate without weakening the virus itself.
Dr. Bert Schepens, Postdoctoral Scientific Staff, Ghent University
The results represent a step toward developing durable and broadly applicable antiviral treatments for COVID-19 and other coronavirus-related illnesses.
The combination of high efficacy, broad activity against multiple virus variants, and a high threshold for resistance is particularly promising. This work lays a strong foundation for the development of new antibodies that could help patients not only today, but also in the future.
Xavier Saelens, Professor, Center for Medical Biotechnology, Vlaams Instituut voor Biotechnologie
Source:
Journal reference:
De Cae, S., et. al. (2025) Ultrapotent SARS coronavirus-neutralizing single-domain antibodies that clamp the spike at its base. Nature Communications. doi.org/10.1038/s41467-025-60250-1.