 By Pooja Toshniwal PahariaReviewed by Lexie CornerJul 11 2025
By Pooja Toshniwal PahariaReviewed by Lexie CornerJul 11 2025A new study in Nature Chemical Biology introduces SNAP-tag2, an enhanced version of the widely used SNAP-tag self-labeling protein (SLP). SNAP-tag2 offers faster protein labeling, improved fluorescence brightness, and better substrate accessibility.
These improvements could make it a more effective tool for live-cell imaging and other bioimaging applications, particularly in tracking proteins and monitoring cellular activity.
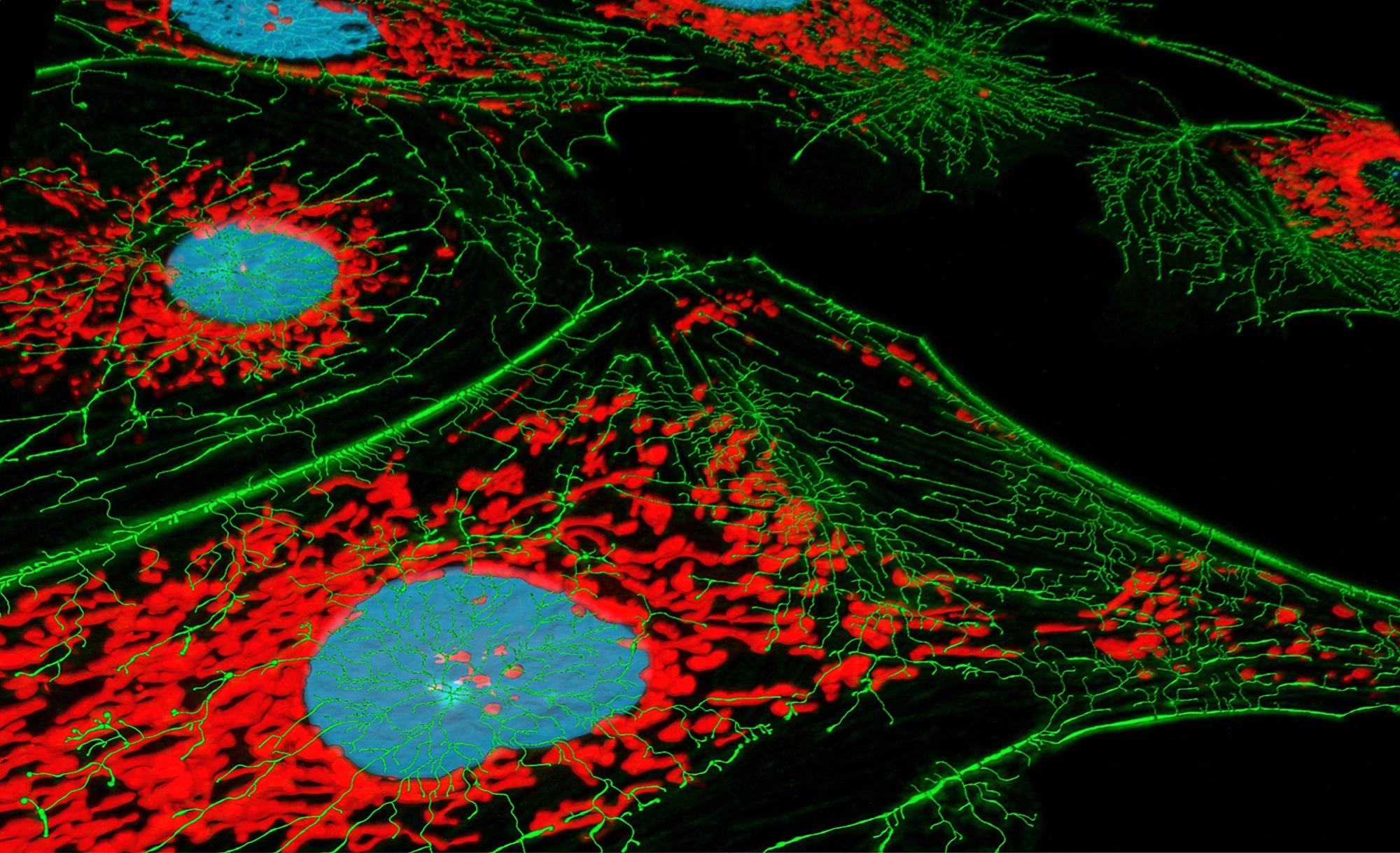
Image Credit: Heiti Paves/Shutterstock.com
Background: Limitations of Current SLP Tools
SLPs have become essential for bioimaging, allowing researchers to label proteins with synthetic probes both in vitro and in live cells. SNAP-tag is a commonly used SLP that enables labeling with synthetic fluorophores.
However, it has drawbacks. Its relatively slow reaction kinetics and limited substrate permeability reduce its effectiveness in live-cell settings. It also performs less efficiently with rhodamine dyes than other tags like HaloTag7.
About the Study
In this study, researchers developed SNAP-tag2, an improved version of the SNAP-tag system, by optimizing both the substrate and the protein itself.
To enhance the intrinsic reactivity and live-cell compatibility of SNAP-tag, they designed several new substrate scaffolds. Seventeen heterocycles were synthesized, each linked via benzyl groups to tetramethylrhodamine (TMR), and tested using fluorescence polarization assays to evaluate reaction kinetics. Among these, trifluoromethyl-substituted pyrimidine (CF3P) showed the best performance, with a threefold increase in labeling speed and a twofold boost in fluorescence in both in vitro and live-cell conditions.
Further cellular assays examined SNAP-tag labeling using 12 linker variants that paired TMR fluorescent probes with chloropyrimidine (CP) leaving groups. Researchers evaluated the substrates’ physicochemical properties—including logP (octanol-water partition coefficient), aqueous solubility, and MDCK permeability—using non-fluorescent N-acetylated protein derivatives. By combining the best features of the CF3P and CF scaffolds, they developed a new compound: trifluoromethyl fluorobenzyl pyrimidine (TF).
On the protein engineering side, the team aimed to improve reactivity and fluorescence with pyrimidine-based substrates when labeled with MaP618, a fluorogenic rhodamine. They employed a combination of computational design, site-specific mutagenesis, and directed evolution to generate mutants with improved fluorogenicity. Deep mutational scanning helped identify which sequence changes had the greatest impact on performance.
Yeast surface display (YSD) combined with fluorescence-activated cell sorting (FACS) was used to screen large libraries for variants with faster TMR labeling kinetics and stronger MaP618 fluorescence. The PROSS algorithm identified stabilizing mutations, while RosettaRemodel replaced a disordered region in the N-terminal domain (NTD) with an eight-residue loop to improve structural stability.
To evaluate SNAP-tag2 in live-cell imaging, the researchers compared its labeling kinetics against SNAPf-tag using various probes—TMR, carbopyronine (CPY), and silicon rhodamine (SiR)—in live U2OS cells. Labeling was quantified through flow cytometry of cells expressing SNAP-tag2 or SNAPf-tag fusion proteins.
They also assessed the fluorescence brightness of SNAP-tag2 in live cells relative to SNAPf-tag and HaloTag7. Confocal and STED microscopy were used to study protein localization and signal strength for fusions like CalR–KDEL (targeting the ER lumen) and TOMM20 (marking the outer mitochondrial membrane). The yeast Hansenula polymorpha was used to test SNAP-tag2 labeling in a system that typically resists chemical tagging.
Results
SNAP-tag2 showed clear improvements over SNAP-tag in both in vitro and live-cell imaging. The second-order rate constant for its reaction with rhodamine substrates approached 10⁷ s⁻¹ M⁻¹—nearly 100 times faster than that of the original SNAP-tag with the same dyes. It also provided a fivefold increase in fluorescence brightness when labeled with highly fluorogenic dyes.
The modified tag retained robust performance even after 11 sequence substitutions that replaced an 18-residue region with a shorter, eight-amino-acid loop. Despite these changes, SNAP-tag2 maintained the same melting temperature of 65 °C as the original version.
Its improved labeling kinetics were observed with both CF- and CP-based substrates. Enhanced absorbance in MaP618-labeled samples suggested a greater tendency for the dye to shift into a zwitterionic, and thus more fluorescent, state.
Live-cell comparisons highlighted clear performance differences between SNAP-tag2 and SNAPf-tag. Labeling with CF-SiR showed a half-labeling time of just 20 minutes to reach fluorescence saturation. With CPY substrates, SNAP-tag2 achieved a fourfold faster labeling rate than SNAPf-tag. In the case of TF-MaP618, it increased fluorescence intensity by fivefold, which is in line with in vitro results.
Confocal and STED microscopy confirmed stronger mitochondrial fluorescence signals with SNAP-tag2 compared to SNAPf-tag. This improvement likely stemmed from a higher photon yield. When expression levels were matched, SNAP-tag2’s signal intensity was comparable to that of HaloTag7.
In yeast (H. polymorpha), which is typically resistant to chemical labeling, SNAP-tag2 delivered stronger labeling efficiency—especially when used with CP-MaP555 substrates—than the original SNAP-tag system.
Download your PDF copy now!
Conclusions
SNAP-tag2 offers clear advantages over the original SNAP-tag, with faster kinetics, stronger fluorescence, and better compatibility with diverse biological systems. These features make it a strong candidate for use in live-cell imaging, biosensor development, and studies requiring precise protein tracking.
Its stable structure also opens up possibilities for further modification, such as creating circular or split versions for more complex sensor designs. These tools could help study protein dynamics and cellular processes both in vitro and in live organisms.
Journal Reference
Kühn, S., Nasufovic, V., Wilhelm, J., et al. (2025). SNAP-tag2 for faster and brighter protein labeling. Nat Chem Biol. DOI: 10.1038/s41589-025-01942-z, https://www.nature.com/articles/s41589-025-01942-z