Thin Layer Chromatography (TLC) is a technique for the analysis of chemical mixtures, widely used in most laboratories. Although it is fairly basic and easy to perform, TLC is at the same time very practical and provides useful information.
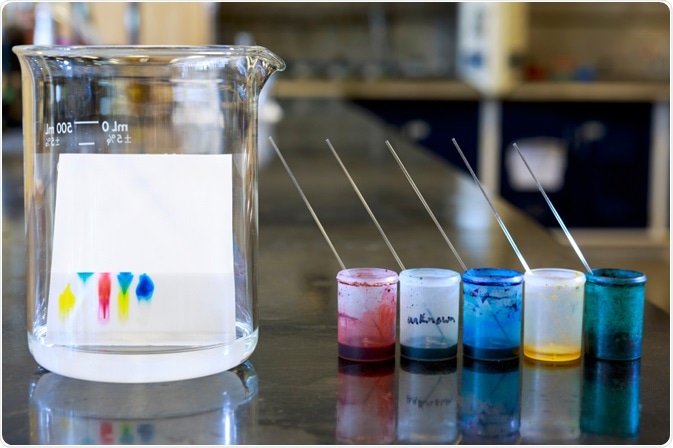
Thin-Layer Chromatography. Image Credit: ggw/Shutterstock.com
The technique is used to determine if a compound is present in a given mixture or to qualitatively assess the purity of a substance. By observing the disappearance of a reagent or the appearance of a product, TLC is often used to monitor chemical reactions.
Some of the key advantages of TLC are the short time required to perform the analysis (typically 5-10 min) and the high sensitivity, with only a few micrograms (10-6 g) of sample needed.
Like other chromatography techniques, TLC requires a mobile phase (eluant) which can be a solvent or mixture of solvents. The stationary phase is typically a plate made of a thin layer of silica gel (100-2000 μm) coated on a solid support (glass or aluminum).
Step-by-step TLC analysis – Development and Visualization
The first step is drawing a baseline with a pencil at 1-1.5 cm from the bottom of the TLC plate, where the sample is “spotted”. Spotting is the term that refers to the application of the sample (previously dissolved in a small amount of solvent) on the TLC plate by using a capillary or a microtube. The plate is then placed in the TLC chamber (i.e., a beaker) containing the mobile phase for the development.
Capillary action makes the eluant travel up the plate and, depending on the intermolecular forces in place, the spotted sample will move up too. There is a competition for the spotted material between the silica and the eluant, with the silica holding the spot in its original place while the solvent tries to move it as it travels up the plate.
Depending on their polarities, different components will move different distances from the original spot, and eventually separate. When the solvent has traveled almost to the top, the plate is removed, the solvent front is marked with a pencil, and the solvent is allowed to dry.
Subsequently, there are several options to visualize the spots. Since most compounds are colorless, the silica gel on the TLC plate contains a fluorescent material that glows under ultraviolet light. Each spot will interfere with the fluorescence and appear as a dark spot on the glowing background.
Alternatively, spots can be visualized by exposing the TLC plate to agents that interact with the organic material on each spot. For instance, iodine vapors and sulphuric acid produce dark-colored spots with most organic compounds, while cerium sulfate is effective with alkaloids, and ninhydrin is used for amino acids.
Once the spots are visualized, it is possible to determine the retention factor (Rf). The Rf value – between 0 and 1 – is an important indicator that it is used to quantify the movement of the materials along the plate. The Rf is obtained by measuring the distance traveled by the substance from the baseline, divided by the distance traveled by the solvent.
If the polarity of the eluant is too high, no separation will occur and all components in the mixture will move along with the solvent (Rf will be too large). Conversely, if the polarity is too low all the spotted components will not move enough (Rf will be too small). Generally, an effective eluant for a good separation should give Rf values in the range of 0.3-0.7.
High‑throughput Analysis and Bioautographic Assay – Other applications of Thin Layer Chromatography
The number of samples that can be spotted on a TLC plate is usually limited. However, there are more complex variants where high-throughput analysis can be performed. An automated 96-well screening platform based on TLC has been developed to monitor the in vitro activity of an enzyme, geranylgeranyl reductase (GGR), isolated from Sulfolobus acidocaldarius (SaGGR).
The screening methodology was used to assess the relative reductase activity of SaGGR towards isoprenoids and the results validated those obtained by GC-MS, which is the routine technique for this type of investigation.
TLC bioautography is a technique that couples TLC separation with in situ estimation of biological activity. The method, originally developed to screen for antimicrobial activity, has now been extended to the determination of inhibitory activity toward enzymes, like neuraminidase (a glycoprotein present in the outermost envelope of influenza viruses), whose inhibitory activity in the roots of Isatis indigotica was identified.
The bioautographic assay is based on the one-step reaction of the enzyme with its substrate and the subsequent formation of blue-colored products. Neuraminidase inhibitory activity was shown by the development of white spots against the blue TLC background.
Interfacing Thin Layer Chromatography with Mass Spectrometry
The combination of TLC with mass spectrometry (TLC-MS), where the spots are analyzed directly from the TLC plate, can be a powerful tool for the investigation of new chemical reactions and the development of novel products.
There are several challenges associated with interfacing TLC with mass spectrometers because, unlike gas or liquid chromatography, after separation, the analyte molecules remain adsorbed on the plate rather than being eluted.
Moreover, the sampling area and the efficiency of extraction and ionization strongly influence the detection limit of TLC–MS. Nevertheless, many TLC-MS techniques have been reported in literature, and some instruments are also commercially available.
Conclusions
It may not be like the other chromatography techniques that enable product purification and characterization, but TLC is without a shadow of a doubt a method that plays an important part. It is already well established and the possibility of performing high-throughput analysis, as well as interfacing with mass spectrometry, consolidates, even more, the role of TLC in every laboratory.
References
- Garabedian, B. M., Meadows, C. W., Mingardon, F., Guenther, J. M., De Rond, T., Abourjeily, R. & Lee, T. S. (2020). An automated workflow to screen alkene reductases using high-throughput thin layer chromatography. Biotechnol Biofuels, 13, 184.10.1186/s13068-020-01821-w
- Zang, Y., Miao, Y., Wu, T. & Cheng, Z. (2020). Development of a thin-layer chromatography bioautographic assay for neuraminidase inhibitors hyphenated with electrostatic field induced spray ionization-mass spectrometry for identification of active Isatis indigotica root compounds. J Chromatogr A, 461597.10.1016/j.chroma.2020.461597
- Cheng, S. C., Huang, M. Z. & Shiea, J. (2011). Thin-layer chromatography/mass spectrometry. J Chromatogr A, 1218, 2700-11.10.1016/j.chroma.2011.01.077
Further Reading
Last Updated: Feb 25, 2021