Retrometabolic approaches have been implemented for both “In Silico” and “wet chemistry” drug design. This systematic methodology is used to provide safe and effective compounds in medicine. This method is a form of retrosynthetic analysis in the sense that it is used to solve difficulties and problems related to organic synthesis.

Image Credit: Gorodenkoff/Shutterstock.com
Drug design of this nature is often delineated by interpreting three-dimensional compounds, investigating key functional groups, and underlining skeletal structures. Some common programs that could be used to perform these feats are PyMOL, Benchling, Geneious, etc.
What is the Nature of Retrometabolic Drug Design?
Many natural compounds are used for medicine, like willow tree bark refined into aspirin or penicillium mold refined to make penicillin. Both compounds are relatively abundant, while their chemical makeup is simple, making them easy to replicate in the lab. However, there are orphan drugs and rare biomarkers that are much more difficult to synthesize and are much less abundant. In situations like these, the implementation of Retrometabolic drug design in the lab is quite feasible. Here, chemists look for a synthetic pathway to build the rare “target molecule.” This is done by breaking the analyte down into smaller and smaller fragments.
This would be akin to completing a maze, though you start from the endpoint rather than the beginning. It would be a moot point to start from an arbitrary molecule and add on moieties so that the end goal will be met. Instead, scientists employ methods of isolating target moieties and functional groups and seeing if these targets can be found in nature or isolated from natural substances.
Retrosynthesis, in this case, is very common amongst organic chemists and can be easily denoted by drawing retrosynthetic arrows.
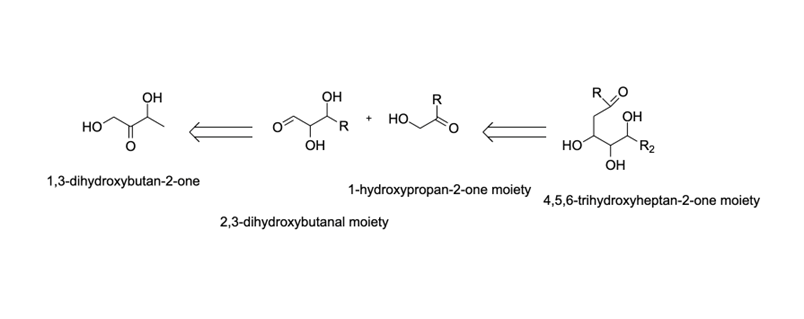
In this example, the identity of all precursors was founded upon the end product. In class and the lab, the chemist uses preliminary knowledge of reaction types, such as nucleophilic substitution, aromatic substitutions, addition reactions, etc., to elucidate a reasonable pathway by which the compound might be enzymatically synthesized.
Examples of some drug variants that are often unearthed through the use of Retrometabolic drug designs are β-blockers, anti-inflammatory steroids, analgesics, and more. Complete libraries of potential drug analogs have been created through hands-on work and in silico design using computer programs and algorithms. Further mathematics and assaying methods have made it possible to rank these candidates using electronegativity, steric strain, and solubility as suitable criteria.
There are many potential reactions that could generate a single functional group. If one wanted to hypothesize the subsequent preliminary reactions to make said functional group, the possibilities would increase exponentially. Therefore, further computation and software are necessary to assess these drug analogs.
How does one Perform Retrometabolic Synthesis?
There are many tools and techniques that one would adopt in an organic chemistry class to allow someone to manipulate and inspect molecular interactions. If one endeavored to solve or understand a retrosynthetic pathway, it would help to count the carbon chain within the final product. From here, any disconnection of any carbon-carbon bonds is accounted for, and the sum of all precursors can add up to the parent molecule. These disconnections, or breaks between molecules, are created through known organic reactions and past experimentation.
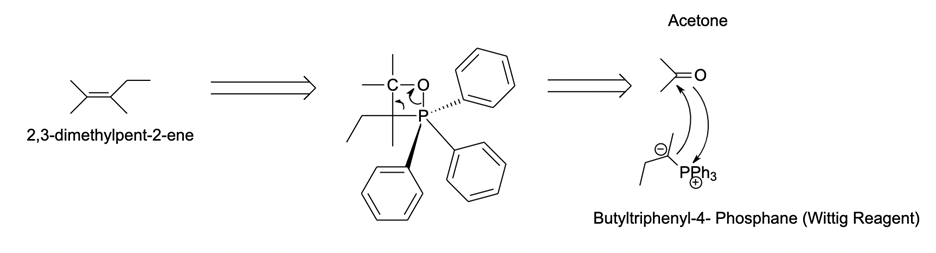
Let’s look at the simple example illustrated above. First, we need to understand what molecule we wish to synthesize, what functional groups are featured in that molecule, and what reactions are required to produce these functional groups. Our goal here is to make the following alkene (2,3-dimethylpent-2-ene) from acetone and a non-specified Wittig reagent.
We know that to form the alkene, the negatively charged carbon on the Wittig reagent and the carbonyl carbon must form a double bond. We know that the three carbons on acetone can generate the left-hand side of the product, which means that a four-carbon fragment would be needed to generate the rest. We know that the proposed reaction above would be valid using all this information.
Here, the nucleophilic addition of acetones C=O bond can form two new bonds, resulting in a strained four-carbon cyclic structure. This transition step has bond angles that approximate 109.5o, and the instability of this ring will cause the fragmentation of oxaphosphetane by eliminating the Ph3P=O. This will result in the desired product alkene.
Retrometabolic Approaches for Drug Design and Targeting
Retrometabolic drug design focuses primarily on improving drug distribution while mitigating toxicity by improving the therapeutic index of the drug in question (activity/toxicity ratio). To enhance these drug features, pharmaceutical scientists look at chemical delivery systems (CDS) and “functional group potency,” both of which are grounded on probable enzymatic activation of specific target sites. A secondary goal of this drug design methodology is the formation of the drug itself regarding how “green” the process is. Like any other protocol performed in the lab, practicing the standards set by green analytical chemistry and sustainable development goals should be of utmost importance.
Sources:
- Bhardwaj YR, Pareek A, Jain V, Kishore D. (2014) Chemical delivery systems and soft drugs: Retrometabolic approaches of drug design. Saudi Pharm J.;22(4):290-302.
- Retrosynthetic analysis and metabolic pathway prediction. (2019). University of Minnesota Morris.
- Bodor N, Buchwald P. (2010) Recent advances in Retrometabolic drug design (RMDD) and development. Pharmazie. Jun;65(6):395-403.
- Dhaneshwar S, Jain A, Tewari K. Design and applications of bioprecursors: a retrometabolic approach. Curr Drug Metab. 2014 Mar;15(3):291-325.
- Tókés B, Suciu G, Nagy G. (2002) Interdependent chemical-electrochemical steps in retrometabolism-based drug and safer chemical design. Pharmazie.
- Prokai-Tatrai K, Prokai L, Bodor N. (1996) Brain-targeted delivery of a leucine-enkephalin analogue by retrometabolic design. J Med Chem. 22;39(24):4775-82.
Further Reading
Last Updated: May 16, 2022