Researchers from the University of Liverpool have provided a new understanding of how cyanobacteria build the organelles that are crucial for their potential to photosynthesize.
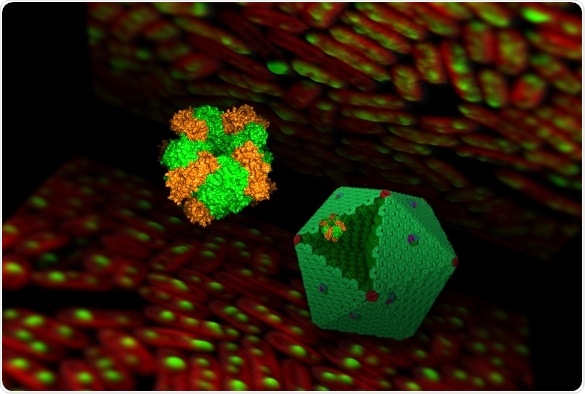
Illustration of a complex carboxysome structure and Rubisco enzymes. Image Credit: Luning Liu.
The study was performed in association with the University of Science and Technology of China, and the results have been published in the Proceedings of the National Academy of Sciences.
Cyanobacteria are an ancient cluster of photosynthetic pathogens that occur in the ocean and also in a majority of the inland waters. These bacteria have evolved a protein organelle, known as the carboxysome, to efficiently change environmental carbon dioxide into sugar.
A crucial step of this conversion process is catalyzed by a carbon-fixing enzyme called Rubisco. But this enzyme is poorly “designed” because it cannot efficiently fix CO2 in the presence of high concentrations of O2.
As a result, cyanobacterial carboxysomes sequester and concentrate the Rubisco enzymes inside the isolated compartment and offer a low O2 setting for the Rubisco enzyme to enhance carbon fixation.
It is a mystery how cyanobacterial cells generate the complex carboxysome structure and pack Rubisco enzymes in the organelle to have biological functions. My research group has interest in addressing the key questions in this biological process.”
Luning Liu, Study Senior Author and Professor, University of Liverpool
The development of the Rubisco complex involves certain “helping” proteins known as chaperones, such as a protein called Rubisco assembly factor 1, or Raf1 for short.
To figure out the precise function of the Raf1, the researchers employed advanced microscopy techniques, like electron microscopy, confocal fluorescence microscopy, and cryo-electron microscopy, integrated with biochemical methods and molecular biology, to explore how the Raf1 communicates with the Rubisco subunits to support the assembly of the Rubisco enzyme and how the formation of carboxysome is affected when cells fail to synthesize the Raf1.
The scientists demonstrated that the Raf1 is essential for constructing the Rubisco complex. If the Raf1 is absent, the Rubisco complexes will assemble in a less efficient manner and cannot be closely packed within the carboxysomes. This could considerably influence the construction of carboxysomes and thus the growth of cyanobacterial cells.
This is the first time that we have determined the function of Rubisco assembly chaperones in the biosynthesis of carboxysomes in cyanobacterial cells. We are very excited about this finding. It also allowed us to propose a new working model of carboxysome biogenesis, which teach us in detail how Rubisco complexes are generated, how Raf1 drive Rubisco packing, and how the entire carboxysome structure is constructed.”
Dr Fang Huang, Study First Author and Leverhulme Trust Early Career Fellow, University of Liverpool
At present, there is an enormous interest in transferring carboxysomes into crop plants to enhance food production and crop yield. This research may offer significant data needed for producing functional and intact carbon-fixing machinery.
Source:
Journal reference:
Huang, F., et al. (2020) Rubisco accumulation factor 1 (Raf1) plays essential roles in mediating Rubisco assembly and carboxysome biogenesis. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2007990117.