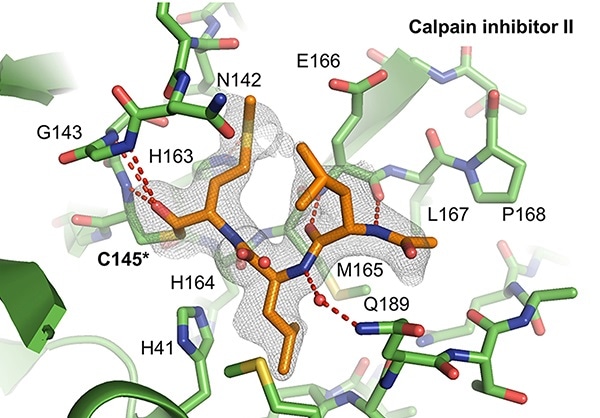
X-ray crystal structures of the SARS-CoV-2 protein Mpro interacting with calpain inhibitors II (depicted above in orange) and XII (below in blue). Calpain inhibitor XII adopts an atypical inverted binding pose. Images Credits: Michael Sacco, University of South Florida Health.
Now, a new research work provides a better understanding of developing antiviral drugs against the COVID-19 infection by demonstrating that certain prevailing compounds can prevent the main protease (Mpro)—an important viral protein that allows the SARS-CoV-2 to replicate within the human cells—and also a human protein, called the lysosomal protease cathepsin L, that is crucial for the virus to penetrate the host cells.
If we can develop compounds to shut down or significantly reduce both processes—viral entry and viral replication—such dual inhibition may enhance the potency of these compounds in treating the coronavirus infection. Metaphorically, it’s like killing two birds with one stone.”
Yu Chen, PhD, Study Co-Principal Investigator and Health Associate Professor of Molecular Medicine, University of South Florida Health
Chen has expertise in structure-based drug design.
Collaborators from the USF Health-University of Arizona (UA) built upon their earlier study, which discovered and investigated many potential prevailing antiviral medications as candidates for treating the COVID-19 disease. All the candidates selected to pursue the Mpro target so as to inhibit the replication of the SARS-CoV-2 virus inside the human cells were cultured in laboratory settings.
A couple of compounds, called calpain inhibitor II and calpain inhibitor XII, did not exhibit as much activity against the Mpro target as another drug candidate, known as GC-376, in biochemical tests. But the calpain inhibitors, particularly XII, in fact, worked better than the GC-376 drug candidate at destroying the SARS-CoV-2 virus in cell cultures, stated Michael Sacco, the study’s lead author and a doctoral student in Dr. Chen’s laboratory.
We figured if these calpain inhibitors were less effective at inhibiting the virus’s main protease, they must be doing something else to explain their antiviral activity,” Sacco said.
Michael Sacco, Study Lead Author and Doctoral Student, University of South Florida Health
The team learned from studies performed by other teams, including Jun Wang, Ph.D., a collaborator and the co-principal investigator of the study from UA, that calpain inhibitors can actually inhibit other proteases, such as cathepsin L—a crucial human host protease that plays a role in mediating the entry of the SARS-CoV-2 into cells.
In the new study, the researchers from the USF Health employed sophisticated methods, specifically X-ray crystallography, to observe how calpain inhibitor II and calpain inhibitor XII, communicated with the viral protein Mpro. As expected, the calpain II inhibitor fits into the targeted binding locations on the surface of the SARS-CoV-2 main protease, the team noted.
Surprisingly, the researchers also found that the calpain XII inhibitor adopted a special configuration—called “an inverted binding pose”—to snugly fit into Mpro active binding sites. (A tight fit improves the interaction of the inhibitor with the targeted viral protein, reducing the enzyme activity that aids in the proliferation of the SARS-CoV-2 virus.)
Our findings provide useful structural information on how we can design better inhibitors to target this key viral protein in the future.”
Yu Chen, PhD, Study Co-Principal Investigator and Health Associate Professor of Molecular Medicine, University of South Florida Health
Apart from the increased potency (the required drug effect at a lower dose) of aiming at the viral protease Mpro as well as the human protease cathepsin L, another advantage of dual inhibitors is their ability to inhibit drug resistance, added Dr. Chen.
SARS-CoV-2 can change, or mutate, its targeted sequence of genes. Such viral mutations dupe the human cell into enabling the virus to bind to the surface membrane of the cell and inject its genetic material. These mutations can also modify the shape of viral proteins and the way they communicate with other kinds of molecules (such as inhibitors) within the cell.
When the virus changes so that it can proceed to reproduce, it can become impervious to a specific inhibitor, decreasing the effectiveness of that specific compound. To put this in simple terms, if the genetic sequence of the viral target (lock) alters, the major (inhibitor) will not fit that particular lock anymore.
However, if it is assumed that the same key can open both locks to help inhibit the COVID-19 disease; in such a case, both locks are the viral target protein Mpro, and the human target protein, cathepsin L.
“It’s harder for the virus to change both locks (two drug targets) at the same time,” added Dr. Chen. “So a dual inhibitor makes it more difficult for antiviral drug resistance to develop, because even if the viral protein changes, this type of compound remains effective against the human host protein that has not changed.”
The researchers from the USF Health-University of Arizona continue to further improve the prevailing antiviral drug candidates to enhance their performance and stability and hope to utilize what they have learned to help develop novel drugs against COVID-19. The next steps of the team will involve solving the structural and chemical interaction of calpain inhibitors with cathepsin L
Source:
Journal reference:
Sacco, M, D., et al. (2020) Structure and inhibition of the SARS-CoV-2 main protease reveals strategy for developing dual inhibitors against Mpro and cathepsin L. Science Advances. doi.org/10.1126/sciadv.abe0751.