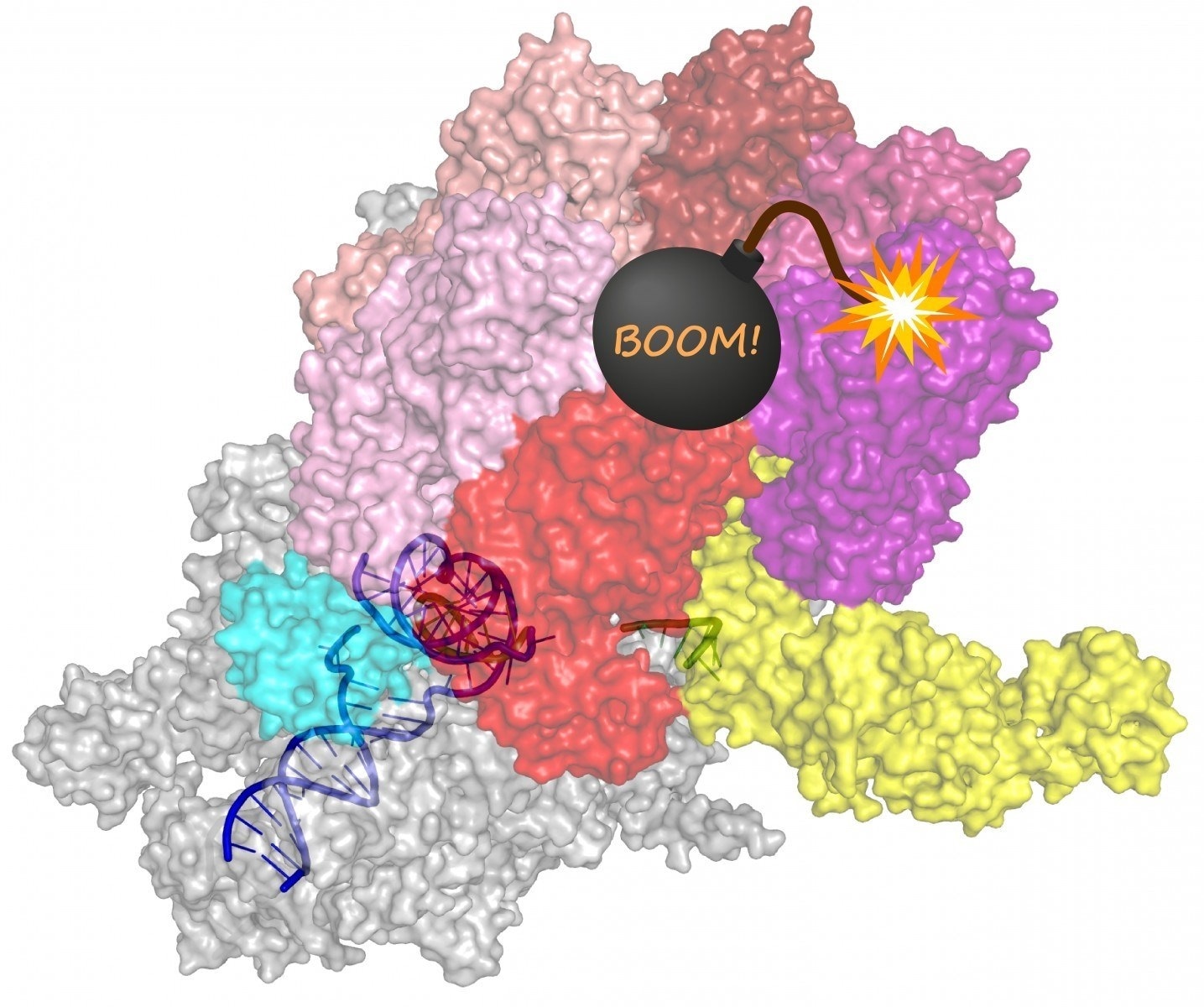
The Rho protein essentially is a sitting time bomb, getting ready to blow the transcription complex to pieces. Image Credit: Irina Artsimovitch.
This latest finding concerns a major process that is integral to life—the transcription stage of gene expression, which allows cells to survive and perform their tasks.
At the time of transcription, an enzyme known as RNA polymerase encases itself around the double helix structure of DNA, utilizing a single strand to correspond with the nucleotides to create a copy of genetic material; this results in a freshly synthesized RNA strand that breaks off upon the completion of transcription. That specific RNA allows the synthesis of proteins, which are crucial to all life and carry out a majority of the work within the cells.
Similar to other coherent messages, RNA should begin and end in the right location to make sense. Rho, a bacterial protein, was identified over five decades ago because of its potential to terminate, or stop, transcription.
According to textbooks, the Rho protein is utilized as a model terminator that uses its extremely strong motor force to attach to the RNA and thus pulls it out of RNA polymerase. However, a closer inspection by these researchers demonstrated that the Rho protein would be unable to detect the RNAs because it needs to discharge using the textbook mechanism.
“We started studying Rho, and realized it cannot possibly work in ways people tell us it works,” stated Irina Artsimovitch, the study’s co-lead author and professor of microbiology at The Ohio State University.
The study determined that rather than binding to a particular part of RNA close to the transcription end and aiding it to unwind from DNA, the Rho protein, in fact, “hitchhikes” on RNA polymerase for the period of transcription.
This protein cooperates with other kinds of proteins to ultimately coax the RNA polymerase via a sequence of structural variations that end with an inactive state, thereby allowing the discharge of the RNA.
The study was recently published online in the Science journal on November 26th, 2020.
The researchers used advanced microscopes to demonstrate how the Rho protein acts on a fully transcription complex made up of RNA polymerase as well as a pair of accessory proteins that travel with it throughout transcription.
This is the first structure of a termination complex in any system, and was supposed to be impossible to obtain because it falls apart too quickly. It answers a fundamental question - transcription is fundamental to life, but if it were not controlled, nothing would work. RNA polymerase by itself has to be completely neutral.”
Irina Artsimovitch, Study Co-Lead Author and Professor of Microbiology, The Ohio State University
Artsimovitch continued, “It has to be able to make any RNA, including those that are damaged or could harm the cell. While traveling with RNA polymerase, Rho can tell if the synthesized RNA is worth making—and if not, Rho releases it.”
In fact, Artsimovitch has made several significant findings on how the transcription is effectively completed by RNA polymerase. She, however, did not set out to challenge decades of understanding about the role of the Rho protein in termination, until an undergraduate student in her laboratory came across unexpected mutations in the Rho protein while working on a genetics study.
It is already known that the Rho protein silences the expression of virulence genes found in bacteria, fundamentally keeping them dormant until they are required to cause infection. However such genes lack the RNA sequences that the Rho protein is known to preferentially attach.
Due to this fact, it has never made sense that the Rho protein looks only for certain sequences of RNA, without even understanding if they are still fixed to RNA polymerase, added Artsimovitch.
As a matter of fact, the scientific interpretation of the mechanism of the Rho protein was established through streamlined biochemical experiments that often overlooked RNA polymerase—essentially, describing how a process ceases without considering the process itself.
In the latest study, the team applied cryo-electron microscopy to record the pictures of RNA polymerase acting on a DNA template in their model system, Escherichia coli. This high-resolution visualization, integrated with high-end computation, enabled precise modeling of transcription termination.
RNA polymerase moves along, matching hundreds of thousands of nucleotides in bacteria. The complex is extremely stable because it has to be— if the RNA is released, it is lost. Yet Rho is able to make the complex fall apart in a matter of minutes, if not seconds. You can look at it, but you can't get a stable complex to analyze.”
Irina Artsimovitch, Study Co-Lead Author and Professor of Microbiology, The Ohio State University
The researchers used an ingenious technique to capture complexes just before they collapsed and eventually observed seven complexes that denote sequential steps in the termination route, beginning from the engagement of the Rho protein with RNA polymerase and ending with a fully inactive RNA polymerase.
The researchers also developed models based on what they observed and subsequently validated the accuracy of these models using biochemical and genetic techniques.
While the research work was performed in bacteria, this termination process may probably take place in other forms of life, added Artsimovitch.
It appears to be common. In general, cells use similar working mechanisms from a common ancestor. They all learned the same tricks as long as these tricks were useful.”
Irina Artsimovitch, Study Co-Lead Author and Professor of Microbiology, The Ohio State University
Source:
Journal reference:
Said. N., et al. (2020) Steps toward translocation-independent RNA polymerase inactivation by terminator ATPase ρ. Science. doi.org/10.1126/science.abd1673.