Improvements in DNA sequencing have revealed a rare syndrome triggered by changes in the SATB1gene. It is hoped that the discovery of this rare genetic syndrome would give information to both individuals and families suffering from this SATB1-syndrome.
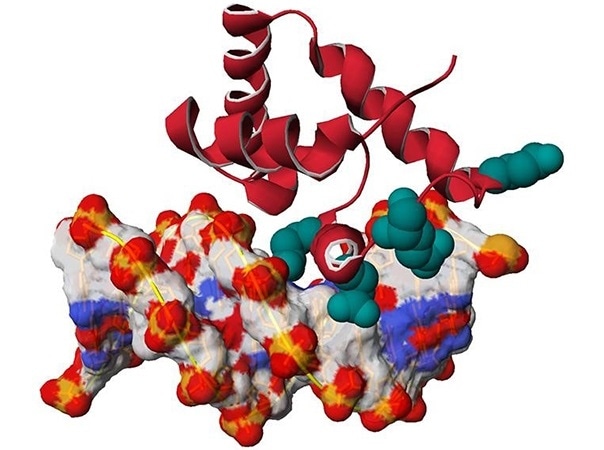
3D model of the SATB1 protein (in red) linked to the DNA double helix (white-red-blue). The positions of mutations found in patients are highlighted in emerald green. Image Credit: © Nicolas Guex, University of Lausanne.
The new study was jointly authored by academics from Oxford Brookes University (United Kingdom), the University of Lausanne (Switzerland), Radboud University (The Netherlands), the University of Oxford (UK), and the University of Manchester (United Kingdom).
Headed by Max Planck Institute for Psycholinguistics (The Netherlands), the study revealed three groups of mutations inside the SATB1 gene, resulting in three differences of a neurodevelopmental disease with differing symptoms spanning from muscle tone abnormalities and epilepsy.
Recognition of disorder will increase understanding and diagnosis
A global team of clinicians and geneticists from 12 nations identified a total of 42 individuals who had mutations in the SATB1 gene and all of them had a host of analogous symptoms, although with varying severity.
It was observed that mutations, or variations, of the SATB1 gene, have different impacts on the cell. For instance, certain mutations resulted in increased activity of the protein which is responsible for causing a more adverse type of disorder, whereas other mutations cause loss of gene function and lead to difficulties that are less severe.
Symptoms of SATB1-syndrome include intellectual disability, neurodevelopmental delay, dental abnormalities, facial dysmorphism, behavioral problems, Epilepsy, and muscle tone abnormalities.
Previously, just one or two cases of patients with SATB1 variations had been described but it was not recognised as a specific syndrome. Patients displaying these characteristics and their families, will have known that they had an undefined neurological condition, but they wouldn’t have known any specific detail about the condition, or why they had it.”
Dr Dianne Newbury, Senior Lecturer in Medical Genetics and Genomics at Oxford Brookes University
Dr Newbury added, “We hope that the recognition of this new disorder, and the information about the molecular pathways contributing to it, will help the families and individuals affected understand more about the condition and achieve a diagnosis they would not have had previously.”
Three classes of mutation have different effects on the cell
It was observed that the mutations detected in the patients’ genome are part of the three different classes of mutation. The first class of mutation, detected in eight individuals, led to a loss of function of the SATB1 gene and reduced the synthesis of the encoded protein by 50%; this results in a less severe syndrome defined by facial dysmorphism, visual problems, and diminished cognitive function.
The second class of mutation includes four types of mutations, which tend to encode shorter proteins but these are less efficient because they are not properly placed in the cell. This class of mutation appears as an intermediary syndrome, which is defined as more intense than the first mutation, but less severe than the third one.
The third class of mutation covers the mutations identified in the last 30 patients. These mutations alter the encoded protein, rendering it more active.
This modified protein is quite “sticky” and attaches better to DNA, reducing the expression of genes it controls and leading to a more serious type of disorder, defined by epilepsy, severe intellectual disability, specific facial features, and a motor speech disorder (also called dysarthria).
Understanding mutations is key to discovering the origin of genetic diseases
These results demonstrate that each mutation is different and that is essential to understand their mode of action in order to explain the origin of genetic diseases. “We must go beyond sequencing, which is only a first step.”
Dr Alexandre Reymond, Director of the Center for Integrative Genomics, University of Lausanne
The study titled “Mutation-specific pathophysiological mechanisms define different neurodevelopmental disorders associated with SATB1 dysfunction” has been published in The American Journal of Human Genetics.
Source:
Journal reference:
Hoed, J, D., et al. (2021). Mutation-specific pathophysiological mechanisms define different neurodevelopmental disorders associated with SATB1 dysfunction. The American Journal of Human Genetics. doi.org/10.1016/j.ajhg.2021.01.007.