Researchers from the Tokyo Institute of Technology (Tokyo Tech) have revealed mechanisms behind the stimulation of the MRN complex—the DNA scissors of the cells.
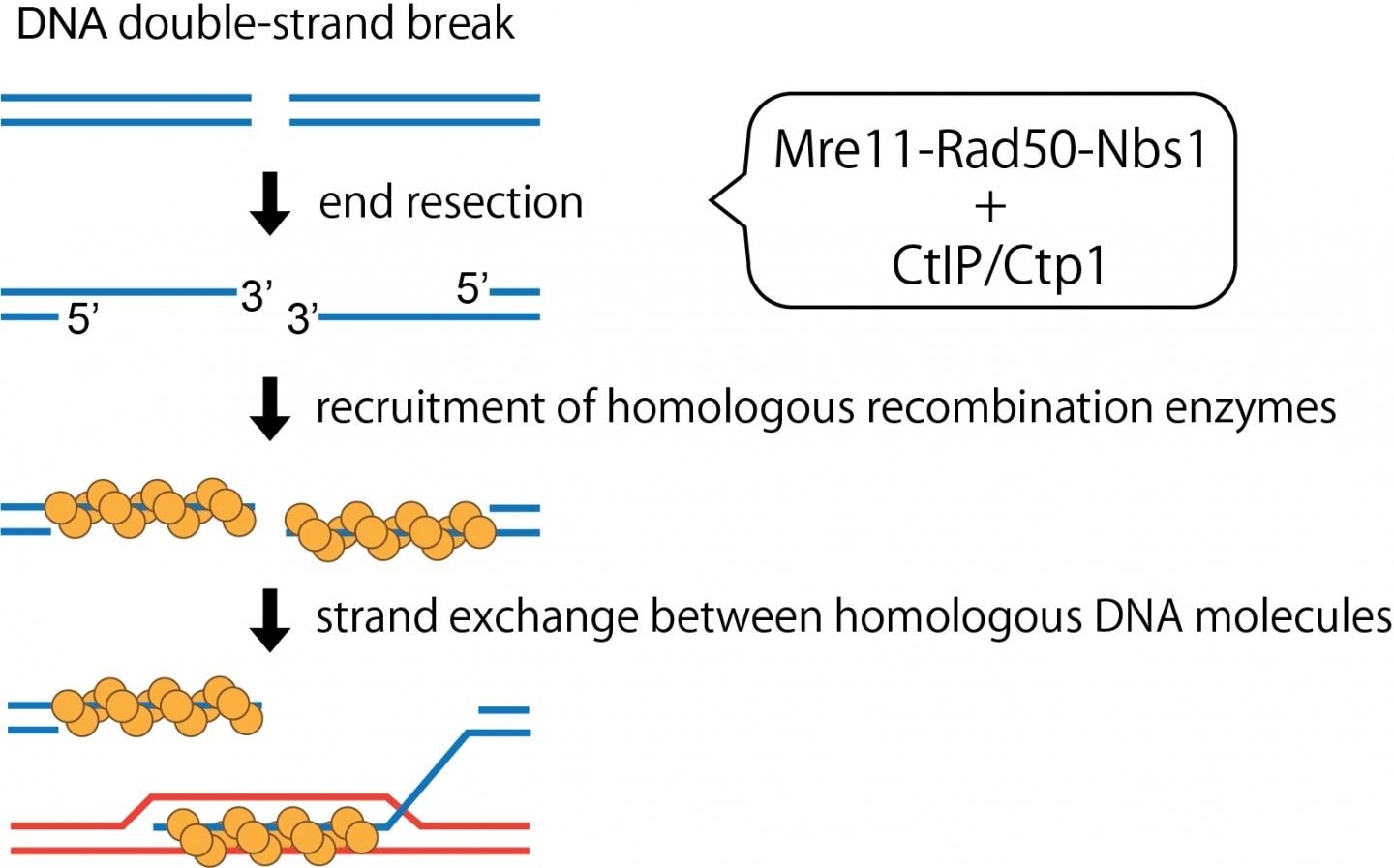
CtIP/Ctp1 stimulates endonuclease activity of the MRN complex, which not only polishes “dirty” ends of DNA breaks but promotes accurate repair of DNA breaks through the homologous recombination mechanism. Image Credit: PNAS.
With the help of purified yeast proteins, the researchers have shown that phosphorylation of Ctp1—a homolog of a tumor-suppressor protein—has a crucial role to play in triggering the DNA clipping activity of the MRN complex. Fascinatingly, a short fragment of Ctp1 or its human equivalent could trigger the endonuclease activity of their respective MRN complexes, indicating its limited function across species.
DNA acts as a roadmap that directs the functions and identity of cells. An anomaly in the DNA can have a seriously damaging effect, leading to the loss or malfunction of important proteins, thus influencing the normal function and viability of cells.
Such glitches usually manifest as double-stranded breaks in the DNA that could occur naturally or from exposure to specific chemicals. To overcome these glitches, the cells have developed unique DNA repair machinery that scans, detects, and corrects the DNA breaks by sealing the gaps. But most often, DNA breaks have “dirty ends” that cannot be directly sealed or ligated because they are unexposed or inhibited by specific proteins or abnormal chemical structures.
Hence, these DNA ends should be initially clipped and then released so that they can be further processed. This end-resection of the DNA breaks is also necessary for them to be precisely restored by homologous recombination. Mre11 is a major player among these nuclease enzymes or molecular scissors.
Mre11 binds with Nbs1 and Rad50 proteins to jointly form the “MRN” complex. The interaction between this complex and the CtIP tumor suppressor protein in human beings has been demonstrated to activate the DNA clipping mechanism of the complex. But the mechanisms fundamental to this relationship are yet to be explored.
Assistant Professor Hideo Tsubouchi and Professor Hiroshi Iwasaki of the Tokyo Institute of Technology and their research team have now decoded the stepwise communication and stimulation of the MRN complex using Ctp1 proteins in yeast, which are homologous to the CtIP protein in humans. The study results have been published in the PNAS journal.
The MRN complex is pivotal in the homologous recombination-mediated repair of DNA double stranded breaks. To better understand how CtIP influences the activity of the MRN complex, we purified yeast proteins and quantified their interactions.”
Hiroshi Iwasaki, Professor, Tokyo Institute of Technology
The researchers observed that phosphorylation or the introduction of phosphate groups to Ctp1 was the first crucial step in the activation of the MRN complex. To be more precise, phosphorylation allowed the physical interaction between Ctp1 and the complex Nbs1 protein, which was crucial for the subsequent stimulation of endonuclease. When the MRN complex was combined with unphosphorylated Ctp1, the DNA clipping activity was very poor.
The team also detected a short range of just 15 amino acids in the C-terminal region of the Ctp1 protein that was crucial for the endonuclease function of the Ctp1-stimulated MRN. Furthermore, a synthetic peptide simulating this region of CtIP or Ctp1 was able to trigger the human MRN complex or yeast, respectively, indicating that the role of C-terminal Ctp1 is probably conserved across species and is the final determinant in the activation of the MRN complex.
Modification of the CT15 peptide can yield a strong activator or potential inhibitor of the MRN complex. Targeting this endonuclease activity can have potentially useful applications in homologous recombination-based gene editing.”
Hideo Tsubouchi, Assistant Professor, Tokyo Institute of Technology
With rapid advances in molecular medicine and recombinant DNA, these results may help geneticists uncover the genomic mysteries and detect the concealed complexities of genetic diseases with greater ease and efficiency in the future.
Source:
Journal reference:
Zdravković, A., et al. (2021) A conserved Ctp1/CtIP C-terminal peptide stimulates Mre11 endonuclease activity. PNAS. doi.org/10.1073/pnas.2016287118.