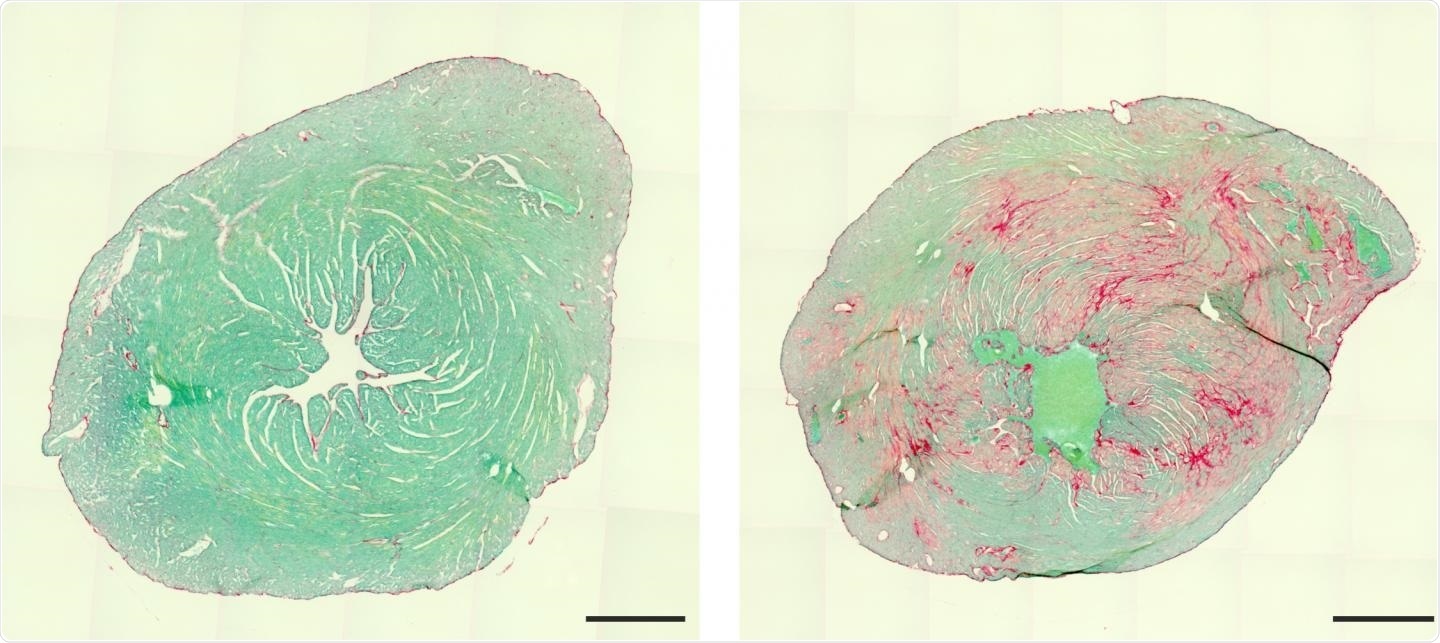
Heart of a healthy (left) and a diseased mouse (cross-sections). Connective tissue inclusions (right, red) make the diseased heart less efficient. Image Credit: Kenichi Kimura/UKB.
In children, the disease leads to severe heart failure and this is accompanied by respiratory and skeletal muscle damage. Those who are infected rarely live past the age of 20. The research also outlines promising therapy options that are being tested in laboratories. But whether this hope will be fulfilled will become evident only after a few years. These latest findings were published in the Nature Communications journal.
Anyone who has broken down with their car or snapped a spoke on their bike would know that mechanical stresses can eventually cause damage that needs to be fixed. This is also true for human musculature.
With each movement, structural proteins are damaged and have to be replaced.”
Dr Michael Hesse, Adjunct Professor, Institute of Physiology, University of Bonn
Hesse led the new study together with his colleague Professor Dr. Bernd Fleischmann.
Normally, the defective molecules are broken down in the cell and their constituents are subsequently recycled. A protein known as BAG3 plays a key role in this complex process. The results of the current study demonstrate the significance of this: The team was able to show that a single alteration in BAG3’s genetic blueprint leads to a deadly disease.
The mutation causes BAG3 to form insoluble complexes with partner proteins that grow larger and larger.”
Dr Michael Hesse, Adjunct Professor, Institute of Physiology, University of Bonn
This halts the repair processes, causing the muscles to become weaker and less efficient. Furthermore, dangerous amounts of proteins build up over time, ultimately causing the death of muscle cells.
The consequences are usually first seen in the heart. There, muscle is successively replaced by scar tissue. This causes the heart's elasticity to decrease until it can barely pump blood.”
Dr Michael Hesse, Adjunct Professor, Institute of Physiology, University of Bonn
As a result, the affected individuals typically need a heart transplant in childhood. Since this condition also affects the respiratory and skeletal muscles, even this measure gives only brief relief. Consequently, patients frequently die at an early age.
A very rare condition, therefore little research
During embryo development, the deadly mutation can emerge spontaneously. Providentially, this is an extremely rare occurrence: only a few hundred children in the world are probably impacted. But due to the rarity of this disease, it has received only minimal attention to far.
“Our study now takes us a great deal further,” said Bernd Fleischmann.
The is because, for the first time, the researchers have successfully simulated the disease in mice and used the novel animal model to uncover its origins. This allows it to be examined more thoroughly than before, including in terms of potential remedies. Perhaps, the impact of the mutation may be mitigated.
Each gene in humans has two copies, one from the mother and one from the father. This implies that even if one form of BAG3 mutates during the development of embryos, a second gene remains unaffected.
Regrettably, the faulty BAG3 also clumps with its intact siblings. As a result, a mutation in one of the genes is sufficient to prevent the breakdown of faulty muscle proteins. However, if the altered version can be removed, the repair should operate properly again. It would also prevent the cell from accumulating so many proteins that it would finally die. But there are techniques for precisely inhibiting the activity of specific genes.
“We used one of them to treat the sick mice,” concluded Kathrin Graf-Riesen, Institute of Physiology.
Graf-Riesen, Dr. Kenichi Kimura, and her colleague Dr. Astrid Ooms were in charge of the majority of the experiments. The animals that were treated in this manner had much fewer symptoms. However, whether this strategy can be applied to people is still being investigated.
Source:
Journal reference:
Kimura, K., et al. (2021) Overexpression of human BAG3P209L in mice causes restrictive cardiomyopathy. Nature Communications. doi.org/10.1038/s41467-021-23858-7.