A group of international researchers generated a three-dimensional (3D) pancreatic cancer tumor model in the lab, merging a bioengineered matrix and patient-derived cells that can be employed to create and test targeted treatments.
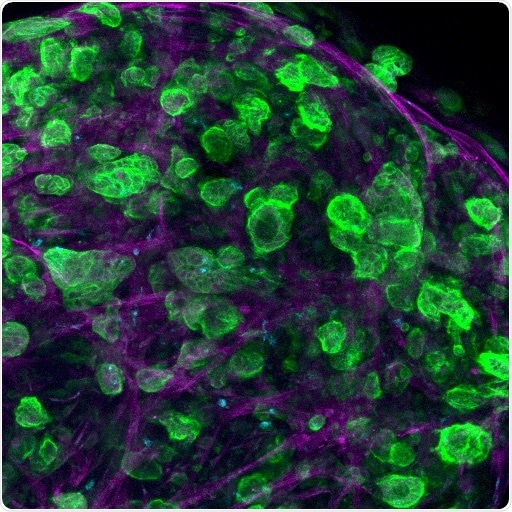
Confocal microscopy image of a triple culture of pancreatic ductal adenocarcinoma (PDAC) cells, macrophages, and pancreatic stellate cells embed and growing within the engineered matrix. Image Credit: Professor Alvaro Mata, University of Nottingham.
In the current research, scientists from the University of Nottingham, Queen Mary University of London, Monash University, and Shanghai Jiao Tong University have developed a multicellular 3D microenvironment that utilizes patient-derived cells to reconstruct the process by which tumor cells develop in pancreatic cancer and their response to chemotherapy drugs.
The research was published on September 24th, 2021, in the Nature Communications journal.
Pancreatic cancer is extremely hard to treat, specifically as there are no signs or symptoms until cancer spreads. It is at times resistant to treatment and has a lower survival rate compared to other cancers—only around 5%–10% survival rate five years after diagnosis.
The research was headed by Professors Alvaro Mata from the University of Nottingham (United Kingdom), Daniela Loessner from Monash University (Australia), and Christopher Heeschen from Shanghai Jiao Tong University (China).
There are two main obstacles to treating pancreatic cancer—a very dense matrix of proteins and the presence of highly resistant cancer stem cells (CSCs) that are involved in relapse and metastasis. In our study, we have engineered a matrix where CSCs can interact with other cell types and together behave more like they do in the body, opening the possibility to test different treatments in a more realistic manner.”
Dr David Osuna de la Peña, Lead Researcher, Queen Mary University of London
There is a demand for enhanced 3D cancer models to examine tumor growth and progression in patients and test responses to new treatments. Currently, 90% of efficacious cancer treatments tested pre-clinically fail in the initial phases of clinical trials and less than 5% of oncology drugs are effective in clinical trials.
Pre-clinical tests generally depend on a combination of two-dimensional (2D) lab-grown cell cultures and animal models to foretell responses to treatment. But traditional 2D cell cultures do not mirror major characteristics of tumor tissues and interspecies differences can affect numerous effective treatments in animal hosts being ineffective in humans.
Eventually, new experimental 3D cancer models are necessary to better reproduce the human tumor microenvironment and integrate patient-specific differences.
Biological systems controllably assemble multiple molecules and cells into functional tissues through a process named self-assembly. Through this mechanism, the researchers developed a new hydrogel biomaterial containing multiple, but specific, proteins present in pancreatic cancer. This process of the formation allows integration of major cell types to generate biological environments that can mimic features of a patient’s tumor.
Using models of human cancer is becoming more common in developing treatments for the disease, but a major barrier to getting them into clinical applications is the turnaround time. We have engineered a comprehensive and tuneable ex vivo model of pancreative ductal adenocarcinoma (PDAC) by assembling and organizing key matrix components with patient-derived cells.”
Alvaro Mata, Professor, University of Nottingham
Professor Mata further states, “The models exhibit patient-specific transcriptional profiles, CSC functionality, and strong tumourigenicity; overall providing a more relevant scenario than Organoid and Sphere cultures. Most importantly, drug responses were better reproduced in our self-assembled cultures than in the other models.”
We believe this model moves closer to the vision of being able to take patient tumor cells in hospital, incorporate them into our model, find the optimum cocktail of treatments for particular cancer and deliver it back to the patient—all within a short timeframe. Although this vision for precision medicine for treating this disease is still a way off, this research provides a step towards realizing it.”
Alvaro Mata, Professor, University of Nottingham
Source:
Journal reference:
de la Peña, D. O., et al. (2021) Bioengineered 3D models of human pancreatic cancer recapitulate in vivo tumour biology. Nature Communications. doi.org/10.1038/s41467-021-25921-9.