Recent research enhances the understanding of certain cancers and complex congenital disorders by illuminating how BRD4—a protein that researchers examined for years—drives the spatial organization of DNA within the nucleus of the cell—a function vital for the differentiation of stem cells into muscle cells.
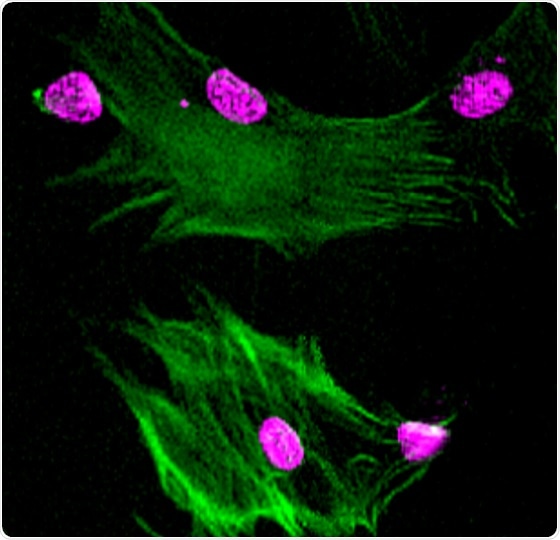
Neural crest progenitor cells were differentiated into smooth muscle cells (green; nucleus of cells stained in pink). Loss of factors underlying genome folding impairs neural crest differentiation into smooth muscle cells. Image Credit: Penn Medicine.
Since the way DNA folds is central to gene regulation, this research has implications that broaden to a range of genetic conditions—including cleft palate, heart defects, microcephaly, and intellectual disabilities—along with better guiding drug target development for BRD4, some of which are in clinical trials as prospective therapeutic interventions to treat heart disease and cancer.
The observations were published on October 6th, 2021, in the journal Nature Genetics.
Inhibiting BRD4 and the class of proteins it belongs to has been seen as a very enticing area of cancer research. A similar strategy is being considered for heart disease patients. Our data highlight the importance of trying to use inhibitors which affect a specific function of BRD4.”
Rajan Jain MD, Study Senior Author and Assistant Professor, Medicine and Cell & Developmental Biology, Perelman School of Medicine, University of Pennsylvania
Rajan Jain further says, “It’s possible that negative results in clinical trials inhibiting BRD4 is because too many functions are being inhibited, so by understanding all the different functions we could be able to eventually have designer drugs which affect one versus another function of BRD4.”
An attractive target, but a poorly understood one
BRD4 is known as a cellular “Swiss army knife” owing to its ability to control multiple facets of how a gene is turned on, along with generally acting as a positive regulator of mechanisms that permit some genes to be switched on.
BRD4 regulates genes including the ones involved in cell growth and division. These genes when switched on abnormally directs certain cancers, including various forms of leukemia. Provided the pro-growth function, inhibition of BRD4 looks like a reassuring strategy to combat those cancers. But, results from certain clinical studies of BRD4 inhibitors proposed that inhibiting BRD4 turns out to be a complicated affair.
Jain’s group demonstrated that BRD4’s function in genome-folding is evident from its function in keeping genes switched on. But, in principle, drugs that attach to BRD4 in specific means can inhibit either or both functions. This means that there is a necessity to consider medicine that targets one function or another of BRD4 and associated proteins.
The inhibitors that block BRD4 are potentially also disrupting the proper folding of DNA in some cells. So I think our work here reveals another layer of complexity to this protein, and should be relevant when researchers are trying to interpret their results with BRD4-affecting inhibitors.”
Rajan Jain MD, Study Senior Author and Assistant Professor, Medicine and Cell & Developmental Biology, Perelman School of Medicine, University of Pennsylvania
Mutations in BRD4 are linked to cohesinopathies—a set of congenital syndromes, like Roberts Syndrome and Cornelia de Lange Syndrome—that gravely weaken and shorten patients’ lives. Cohesinopathies arise due to the dysfunction of cohesin, a multi-protein molecular machine that enables DNA folding and coiling within cells.
The involvement of BRD4 in the workings of cohesin has been greatly unknown, recently. In the current research, scientists identified that loss of BRD4 in the neural crest, a subset of greatly specialized cells that transform into tissue in the heart, face, brain, and other organs, resulted in embryos that share features equivalent to those found in human cohensinopathies.
Jain and his co-workers hypothesize that BRD4 instructs new cells how to fold their DNA so that the correct genes for that cell type are turned on, and anticipate to prove or disprove that in their upcoming study.