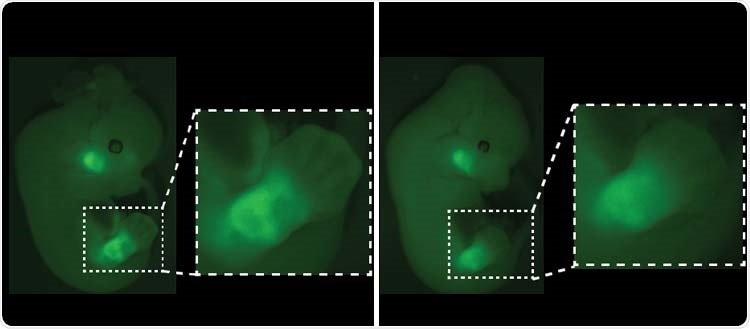
Fluorescence visualization of Pitx1 gene expression in mouse embryos. The absence of Pen, one of the Pitx1 switches (right), results in a loss of fluorescence in developing legs. Image Credit: © University of Geneva/Guillaume Andrey.
By analyzing the Pitx1 gene—one of the genes concerned in the construction of the lower limbs—a group of researchers from the University of Geneva (UNIGE), in Switzerland, identified how a minor disturbance in the activation mechanism of this gene triggers the origin of clubfoot, a common foot malformation.
A wholly functional gene does not act properly in the absence of its genetic switches. These small DNA sequences are vital for this process and offer the signal for the transcription of DNA into RNA. When one of these switches is absent, the proportion of cells where the gene is active rescues, hindering proper development of the lower limbs.
The observations emphasize the greatly undervalued role of genetic switches in developmental disorders. The findings are published in the Nature Communications journal.
At the time of embryonic development, numerous genes should be activated accurately or repressed for organs to form properly. This activity control is regulated by small DNA sequences that, by attaching to specific proteins in the cell nucleus, that function as true ON/OFF switches.
When the switch is turned on, it initiates the transcription of a gene into RNA, which in turn is translated into a protein that can then perform a specific task. Without this, genes would be continuously switched on or off, and therefore unable to act selectively, in the right place and at the right time.”
Guillaume Andrey, Professor, Department of Genetic and Developmental Medicine, Faculty of Medicine, University of Geneva
Guillaume Andrey headed the study.
Normally, every gene has numerous switches to assure that the process is robust.
However, could the loss of one of these switches have consequences? This is what we wanted to test here by taking as a model the Pitx1 gene, whose role in the construction of the lower limbs is well known.”
Raquel Rouco, Study Co-First Author and Post-Doctoral Researcher, University of Geneva
Raquel Rouco is a researcher at Guillaume Andrey’s lab.
A decrease in cellular activation that leads to clubfoot
To achieve this, researchers modified mouse stem cells employing CRISPR-CAS 9, the genetic engineering tool, which facilitates the addition or removal of specific elements of the genome.
Here, we removed one of Pitx1’s switches, called Pen, and added a fluorescence marker that allows us to visualize the gene activation. These modified cells are then aggregated with mouse embryonic cells for us to study their early stages of development.”
Olimpia Bompadre, Study Co-First Author and Doctoral Student, University of Geneva
Generally, around 90% of cells in the future legs activate the Pitx1 gene, while 10% of cells do not.
Guillaume Andrey elaborates, “However, when we removed the Pen switch, we found that the proportion of cells that did not activate Pitx1 rose from 10 to 20%, which was enough to modify the construction of the musculoskeletal system and to induce a clubfoot.”
There was a rise in the proportion of inactive cells in the immature cells of the lower limbs and also the irregular connective tissue—a tissue vital for the development of the musculoskeletal system.
The same mechanism in many genes
Besides the Pitx1 gene and clubfoot, the UNIGE researchers also identified a general principle, whose process can be seen in a huge number of genes. Defective genetic switches can be the start of various malformations or developmental diseases. Furthermore, a gene does not regulate the development of organs in the body, however is generally concerned with the construction of a broad range of organs.
The authors state, “A non-lethal malformation, such as clubfoot, for example, could be an indicator of disorders elsewhere in the body that, while not immediately visible, could be much more dangerous.”
They added,“If we can accurately interpret the action of each mutation, we could not only read the information in the genome to find the root cause of a malformation but also predict effects in other organs, which would silently develop, in order to intervene as early as possible.”
Source:
Journal reference:
Rouco, R., et al. (2021) Cell-specific alterations in Pitx1 regulatory landscape activation caused by the loss of a single enhancer. Nature Communications. doi.org/10.1038/s41467-021-27492-1.