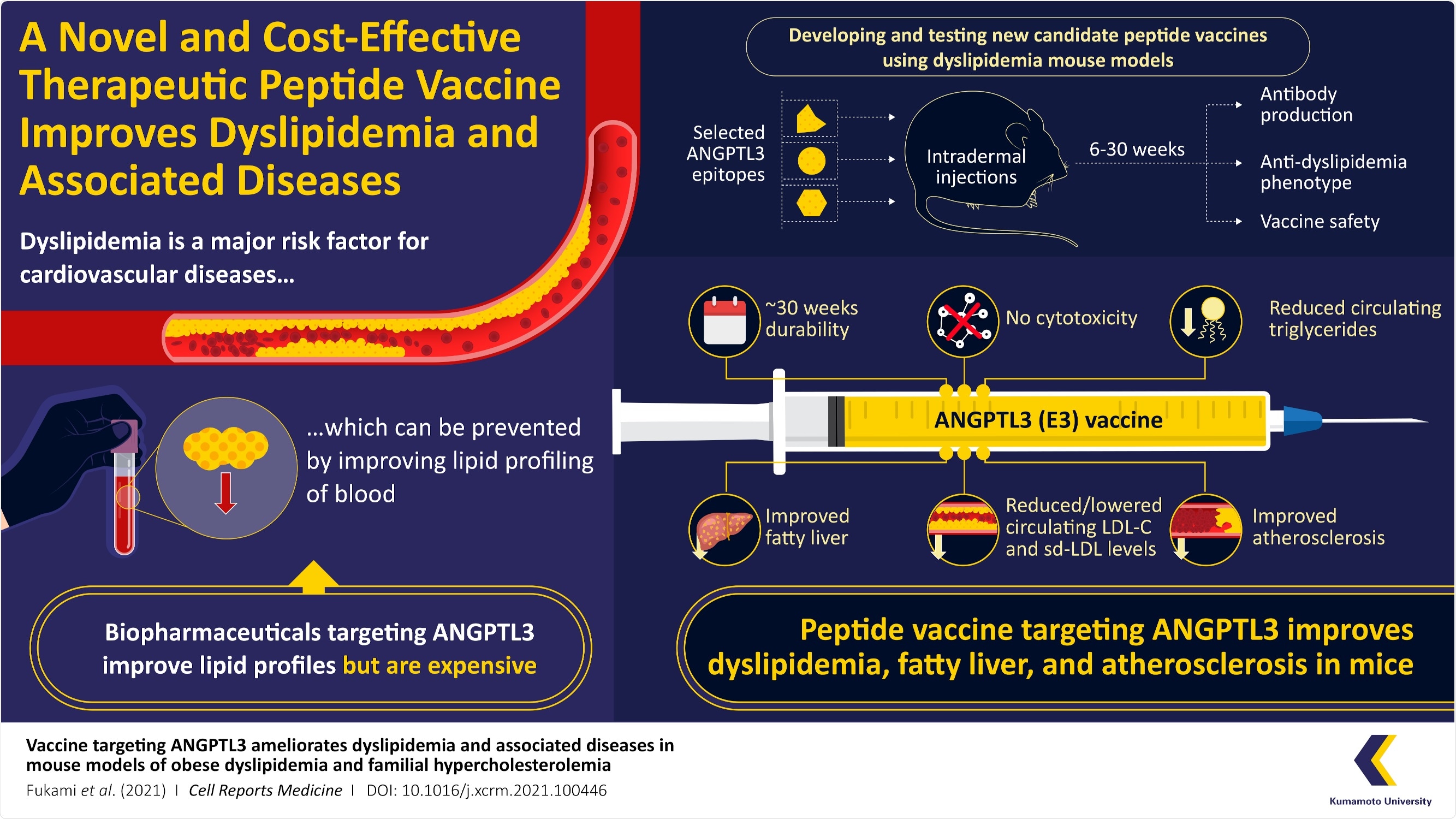
The major risk factor for cardiovascular disease is dyslipidemia, which substantially decreases life expectancy. Professor Yuichi Oike and his group of researchers from the Kumamoto University, Japan, recently produced a peptide vaccine that mitigates conditions of dyslipidemia. Its efficiency was demonstrated in mice.
The new peptide vaccine from researchers at Kumamoto University targets ANGPLT3 and improves dyslipidemia, fatty liver, and atherosclerosis in mice. Image Credit: Kumamoto University.
The novel vaccine can become one of the most inexpensive treatments for obesity/cholesterol-related disorders such as fatty liver disease and atherosclerosis.
Nearly 32% of the global fatalities are implicated by cardiovascular disease (CVD). Dyslipidemia is characterized by higher levels of low-density lipoprotein-cholesterol (LDL-C) and triglycerides (TG), a significant risk factor for CVD. These accelerate the advancement of fatty liver disease, which leads to liver cancer eventually.
As the global frequency of fatty liver disease is high, the market will be soon filled with costly drugs called “biopharmaceuticals” that are now under clinical trial. However, these drugs need long-term treatment, a major hurdle to global healthcare coverage. Hence, researchers are concentrating their efforts on producing novel therapeutic agents that are both inexpensive and efficient.
Professor Yuichi Oike and his group produced a novel peptide vaccine to address obesity-linked dyslipidemia and analyzed its impact on obese mice models. The study was published on November 16th, 2021, in Volume 2 Issue 11 of the journal Cell Reports Medicine.
To produce the vaccine, the researchers chose target angiopoietin-like protein 3 (ANGPTL3), which recently garnered attention in strategies focused on reducing TG and LDL-C levels in the blood. The scientists evaluated the results of the ANGPTL3 peptide vaccine in their trials on mice, which affirmed efficiency for approximately six months with no reports of toxicity, providing evidence that the new treatment is safe and does not have side effects.
Peptide vaccines are inexpensive to manufacture compared to biopharmaceuticals, making them a better choice in terms of the medical economy. The researchers created vaccines targeting three peptides (short chains of amino acids) from ANGPTL3—called epitope 1 (E1), E2, and E3.
These peptide vaccines were later injected into a mouse model of obesity-induced dyslipidemia. The scientists were excited to notice that the E3 peptide vaccine efficiently decreased dyslipidemia in the mice and developed antibodies that lasted up to six months.
The peptide vaccine selectively induces antibody-mediated immunity to produce neutralizing antibodies suppressing ANGPTL3 function.”
Yuichi Oike, Professor, Kumamoto University
As the LDL-Cs get deposited easily in the walls of blood vessels and hinder blood flow, they are generally indicated as “bad cholesterol”. This hindrance causes atherosclerosis and later results in heart failure. To examine if the vaccine was effective against this condition, the scientists administered E3 into familial severe hypercholesterolemia model mice with a high-cholesterol diet and found that it enhanced atherosclerosis and dyslipidemia.
Professor Oike details on the mode of action of vaccine and if the benefits in humans thus, “E3 vaccination in mice decreases circulating levels of LDL-C and TG in the blood. Since the mouse E3 sequence is identical to the corresponding human sequence, E3 could potentially be investigated in clinical studies”.
Professor Oike and his coworkers assume that this vaccination would help patients in economically disadvantaged areas who might need long-term therapy for dyslipidemia or related conditions.
Expensive antibody treatments for dyslipidemia require monthly injections, whereas our vaccination is effective for up to 6 months. The vaccine is projected to become one of the new economical therapy choices for dyslipidemia and dyslipidemia-related disorders.”
Yuichi Oike, Professor, Kumamoto University
The scientists intend to carry out preclinical research to analyze the vaccine's efficiency and safety, and also make developments to extend its efficiency.
We will modify the vaccine formulation and optimize the dosage and administration for clinical trials. Re-immunization every five or six months could maintain the therapeutic efficacy of the E3 vaccine therapy, and more investigation is necessary to see if a vaccination booster would extend its durability.”
Yuichi Oike, Professor, Kumamoto University
Source:
Journal reference:
Fukami, H., et al. (2021) Vaccine targeting ANGPTL3 ameliorates dyslipidemia and associated diseases in mouse models of obese dyslipidemia and familial hypercholesterolemia. Cell Reports Medicine. doi.org/10.1016/j.xcrm.2021.100446.